Utah Education Network
Uen: Atom in a Bag
Learners will use bags of beads with known quantities of electrons, neutrons and protons to identify the element that they represent.
Ducksters
Ducksters: Science for Kids: The Atom
Kids learn more about the science of the atom. Electrons, neutrons, and protons make up the smallest bits of matter.
Environmental Chemistry
Environmental chemistry.com: Anatomy of an Atom
Explains the basics of atomic structure, from simple definitions to information about quantum theory. Accurate and helpful basics whether or not you need the more advanced information.
Sophia Learning
Sophia: The Atom: Lesson 2
This lesson will illustrate that an atom is mostly empty space and has a positively charged, massive core (containing both protons and neutrons called the nucleus) surrounded by negatively charged electrons. It is 2 of 3 in the series...
Sophia Learning
Sophia: Subatomic Particles: The Electron: Lesson 3
This lesson will explain that electrons are negatively charged particles with negligible mass and are found in pairs in orbitals surrounding the nucleus of an atom. It is 3 of 3 in the series titled "Subatomic Particles: The Electron."
California State University
Csudh Project for Chemistry: Protons, Electrons, and Neutrons
This page is an exercise in relating the number of protons, electrons, and neutrons for an atom or monoatomic ion.
CK-12 Foundation
Ck 12: Plix: Build Some Helium: Atoms to Molecules
[Free Registration/Login Required] Build your own helium atom and make sure it has the correct number of protons, electrons, and neutrons on this site. Site also includes a small quiz on the topic.
Estrella Mountain Community College
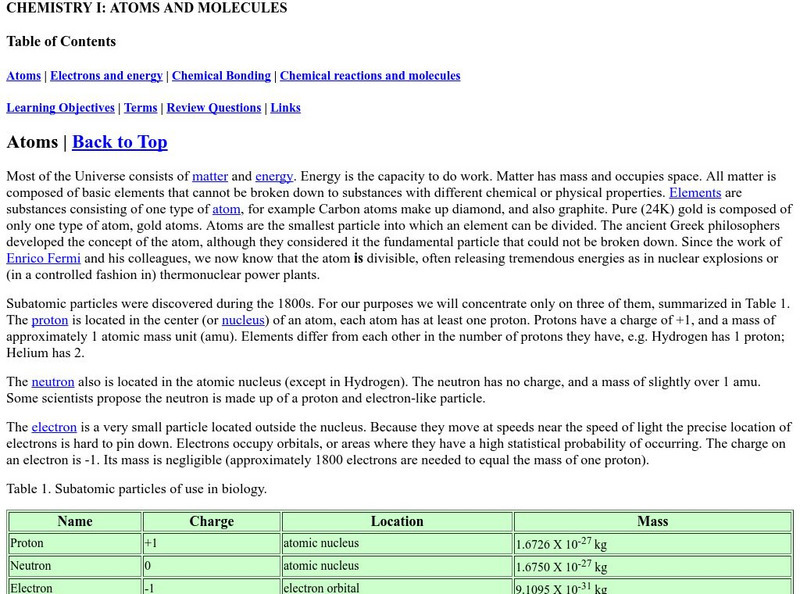
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.
Science Education Resource Center at Carleton College
Serc: Drawing Atoms
This activity serves as an introduction to chemistry, and can be used to help young scholars draw a two dimensional image of the atom.
Science Struck
Science Struck: What Makes Up an Atom?
Describes the structure of an atom and the characteristics of the electrons, neutrons, and protons inside it. Includes some interesting facts about atoms.
Mocomi & Anibrain Digital Technologies
Mocomi: Structure of an Atom
An atom is made of three parts - protons, neutrons, and electrons. Here you can explore these different parts.
Other
Nuclear Twin: The Discovery of the Proton and Neutron
Trace the history of the discovery of protons and neutrons in this informative site.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: All About Atoms
The three basic particles that make up atoms are defined and illustrated.
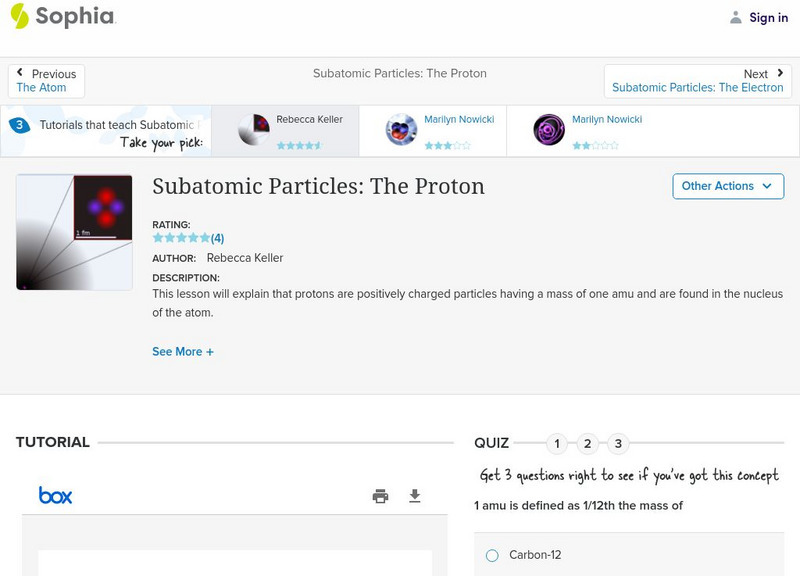
Sophia Learning
Sophia: Subatomic Particles: The Proton: Lesson 2
This lesson will explain that protons are positively charged particles having a mass of one amu and are found in the nucleus of the atom. It is 2 of 3 in the series titled "Subatomic Particles: The Proton."
Science Education Resource Center at Carleton College
Serc: Tic Tac Toe Pick 3 in a Row Atomic Model Assignment
A choice menu assignment where young scholars select between several extension activities that help understand the structure of the atom.
Science4Fun
Science4 Fun: What Is Atom
Fun and interesting illustrated information on atoms including composition, elements, and history.
PBS
Pbs Learning Media: Atomic Structure
Take a look at the parts of an atom and learn about its properties.
Annenberg Foundation
Annenberg Learner: Interactives: The Periodic Table
An interactive website where students learn about the basics of an atom, periodic tables organization, and the structure and properties of matter. Module includes an introduction and five lessons that are followed by a quiz and an...
ClassFlow
Class Flow: Atomic Structure: Parts of the Atom
[Free Registration/Login Required] This flipchart defines the parts of the atom. It uses Activotes for assessment.
ClassFlow
Class Flow: Intro to Atoms
[Free Registration/Login Required] This flipchart was converted from Power Point and is used to introduce the history and concept of the Atom.
Concord Consortium
Concord Consortium: Stem Resources: Atomic Structure
Introduces learners to atomic models of the past and present, focusing on the orbital model and an explanation of its basis. Learners then have the opportunity to "make an atom" and contrast it with an ion, followed by an isotope. The...
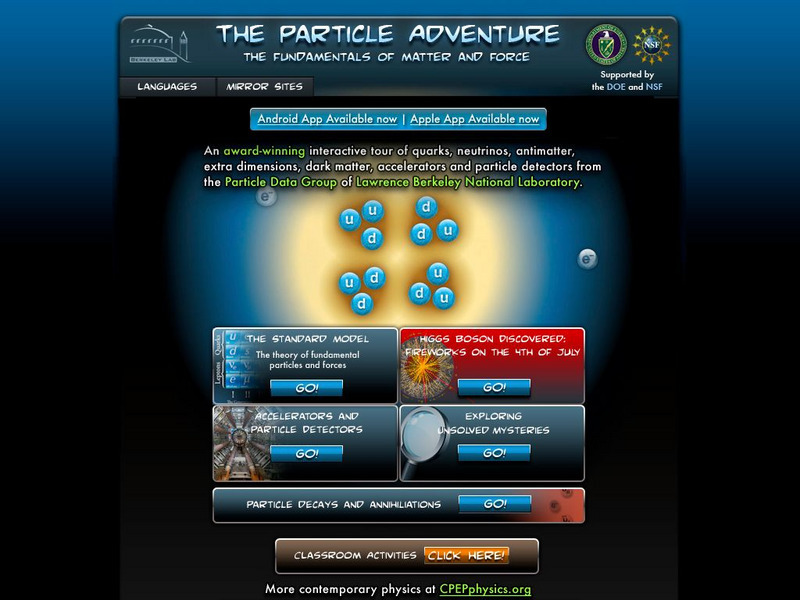
Lawrence Berkeley National Laboratory
Berkeley Lab: The Particle Adventure
Visit this site for an interactive tour of the atom and all aspects of particle physics. View the animations available with almost every description on this site. A great place for the fundamentals of particles and forces including a...
Department of Defense
Do Dea: Chemistry Review
Review atoms with this slideshow. You can choose to listen to the review or read the accompanying text. At the end, play the game to review the basics of atoms that you have just learned about. You may play the game as many times as...
BBC
Bbc: Gcse Bitesize: What Does the Periodic Table Tell Us About the Elements?
The number of protons in the atom of an element determines its place in the Periodic Table. The number of electrons in an atom is the same as the number of protons. These electrons are arranged in shells or 'energy levels' around the...