Simon Fraser University
Chem1 Virtual Textbook: Titration
Acting as an overview from the General Chemistry Virtual Textbook, this site focuses on titration in the relation between acids and bases. The information includes specific mention of titration curves.
Cosmo Learning
Cosmo Learning: General Chemistry
A collection of video lectures from a general chemistry course taught at the University of California, Berkeley. The course teaches periodic table, chemical bonds, molecular shape, phase changes, chemical reactions, stoichiometry,...
Cosmo Learning
Cosmo Learning: Chemistry 1 A: General Chemistry
A collection of video lectures from a general chemistry course taught at the University of California, Berkeley. The course covers topics like stoichiometry, acid-base and solubility equilibrium, oxidation-reduction reactions, chemical...
Chemistry Collective
Chem Collective: Standardization of Na Oh With a Khp Solution: Acid Base Titration
Use the Virtual Laboratory to standardize an unknown NaOH solution (approximately 0.2M) to four significant figures via titration with 25.00 mL of a KHP standard solution.
Chemistry Collective
Chem Collective: Virtual Lab
Perform virtual lab experiments safely. Pour and mix specific amounts of various solutions on screen and observe changes in temperature and pH. Choose glassware and other tools (ie. bunsen burner, scale) to assist with virtual lab...
Chemistry Collective
Chem Collective: Virtual Lab: Default Virtual Lab Stockroom
Perform virtual lab experiments safely. Pour and mix specific amounts of various solutions on screen and observe changes in temperature and pH. Choose glassware and other tools (ie. bunsen burner, scale) to assist with virtual lab...
TeachEngineering
Teach Engineering: Red Cabbage Chemistry
Students take advantage of the natural ability of red cabbage juice to perform as a pH indicator to test the pH of seven common household liquids. Then they evaluate the accuracy of the red cabbage indicator, by testing the pH of the...
Simon Fraser University
Chem1 Virtual Textbook: Acetic Acid
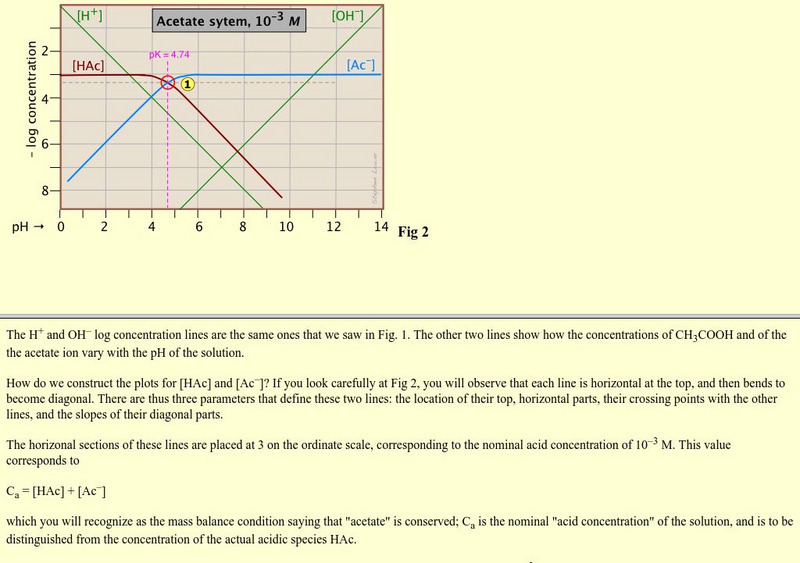
Acting as an overview from the General Chemistry Virtual Textbook, this site acts as one in a series of slides on acid-base systems. This particular slide deals with constructing the plots for acetic acids and graphs.
ClassFlow
Class Flow: Grade or No Grade
[Free Registration/Login Required] This is a game format activity based on the TV game Deal or No Deal. The game is Grade or No Grade. Students answer a series of questions on Chemistry topics of Acids, Bases and Solutions.
Other
Chemistry Tutorials and Simulations
This site includes brief Chemistry tutorials on how to simulate experiments illustrating a number of different topics.
Science Buddies
Science Buddies: Make Your Own P H Paper
In this "kitchen chemistry" project about acid/base chemistry, you will measure the acidity or alkalinity of a solution using a logarithmic scale called the pH scale. As you learn about the pH scale, you will have the chance to make your...
CK-12 Foundation
Ck 12: The P H Concept
[Free Registration/Login may be required to access all resource tools.] In this module, students will define pH and use the hydrogen or hydroxide ion concentrations to calculate the pH of a solution. They will also differentiate among...
Khan Academy
Khan Academy: Types of Catalysts
What is a catalyst? This Khan Academy resource includes examples of enzymes, acid-base catalysis, and heterogeneous (or surface) catalysis.
Khan Academy
Khan Academy: Test Prep: Mcat: Chemical Processes: Chemistry of Buffers and Buffers in Our Blood
Explains what a buffer is, how it is prepared, how it works to resist drastic changes in pH, and the characteristics of an effective buffer. Human blood also contains a buffer which is crucial for maintaining the blood's pH.
University of Arizona
University of Arizona: Problemas De Acidos Y Bases
Learn about the solvent properties of water, pH, pKa and buffering capacity with a series of problems. The correct answers for the multiple choices problems are reinforced with a brief explanation and the incorrect answers are linked to...
Chemistry Collective
Chem Collective: Designing a Buffer Solution With a Specific P H
Create a buffer solution at a specific pH using a weak acid and its conjugate base.
Khan Academy
Khan Academy: Water Autoionization and Kw
Learn about the autoionization of water, the autoionization constant Kw, and the relationship between [H+] and [OH-] in aqueous solutions.
TeachEngineering
Teach Engineering: Engineering Out of Harry Situations
Under the "The Science Behind Harry Potter" theme, a succession of diverse complex scientific topics are presented to students through direct immersive interaction. Student interest is piqued by the incorporation of popular culture into...
Chemistry Collective
Chem Collective: Determining the Concentration of an Unknown Solution
Using the Virtual Laboratory design, students perform an experiment to determine the concentration of the unknown HCl solution to four significant figures.
Chemistry Collective
Chem Collective: Determining Reagent Concentrations
Using the Virtual Laboratory, design an experiment to quickly determine which of the two reagents, HCl or NaOH, is 10 times more concentrated than the other.
University of Nebraska
Do Chem: Simple Heat of Acid Base Reaction
A simple lab activity that allows good data collection on heat of solution.
Purdue University
Purdue University: Calcium Hydroxide Crystal Structure
A picture of the crystal structure of calcium hydroxide. Additional plug-in required.
Open Curriculum
Open Curriculum: Amphoteric Substances
The objective of this article is to introduce amphoteric substances in the context of acid-base chemistry.
Science Struck
Science Struck: The Various Types of Chemical Reactions
A detailed description of the different types of chemical reactions in organic and inorganic chemistry.