Curated OER
A Solution for Precipitation
Students predict the product of chemical reaction using the solubility rules. In this chemistry lesson, students balance ionic equation. They perform a lab to check if their predicted products are correct.
Curated OER
Combustion Reactions
Pupils write a balanced chemical reaction. They articulate how burning fossil fuels increase the carbon dioxide levels in the atmosphere. Students incorporate all the environmental risks involved as well.
Curated OER
Oxidation and Combustion: Chemical Reactions in Fire
Students study oxidation and define vocabulary pertinent to it. In this oxidation lesson students make predictions and create experiments
Curated OER
Activity #6 How Fast Do Reactions Go?
Young scholars name the factors (concentration, temperature, and a catalyst) which can affect the rate of a chemical reaction. They use their knowledge of particle theory to explain their observations. Pupils comprehend that the...
Curated OER
Electroplating for Corrosion Protection: Redox in Action
Students define what a redox reaction is. In this chemistry lesson, students electroplate some wires in the lab. They research the application of electroplating in the real world.
Curated OER
Where My Peeps At?
Students conduct a series of activity that demonstrates Charles' and Boyle's Law. In this chemistry lesson plan, students determine the relationship among pressure, volume and temperature. They solve problems using mathematical...
Curated OER
Rusting: A Form of Oxidation
Learners observe and record the corrosive nature of oxidation-reduction reactions, and determine the electro-chemical series of selected metals.
Curated OER
Individual Gas Laws
Students learn the relationships between the different properties of a gas and use previous knowledge and make predictions. They predit and explain certain phenomena of gases using their chemical knowledge.
Curated OER
Empirical Formulas
In this empirical formulas worksheet, students read about determining empirical formulas from chemical formulas. They determine the empirical formula given ten molecular formulas. They also find the molecular formulas given the mass and...
Curated OER
Identifying Reaction Types
In this reactions learning exercise, students are given nine chemical reactions to identify as single replacement, double replacement, synthesis or decomposition reactions.
Curated OER
Six Types of Reactions
For this types of chemical reactions worksheet, students fill in 5 blanks with the proper type of chemical reaction described in the sentence. Students balance 5 equations and identify the type of chemical reaction for each equation.
Curated OER
Practice Exam III
In this chemistry practice exam worksheet, students solve ten multiple choice questions about equilibrium, acids, bases and reactions. Students then solve four problems about equilibrium and chemical reactions of acids and bases.
Curated OER
Ammonium Nitrate- Stoichiometry
Students quantify the relationship between moles and mass. They comprehend that ammonium nitrate is used as a fertilizer and an explosive. Students study the chemistry of ammonium nitrate and consider the advantages and disadvantages of...
Curated OER
How Can We Calculate an Equilibrium Constant?
Students explain how equilibrium quantities are changed by temperature, pressure or different concentration of substances. They work together to complete equilibrium equations. They identify the relationship between absorption of light...
Curated OER
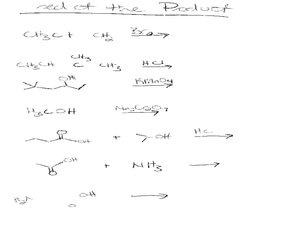
Inorganic Reactions
In this inorganic reactions worksheet, learners complete 7 chemical reactions by predicting the products and balancing the equations.
Curated OER
Solubility Homework Problem Set
In this chemistry activity, students write the balanced equation for the reaction and rearrange the potassium expression to solve for x. Then they determine how many grams of silver chromatic can dissolve in a specified amount of sodium...
Curated OER
Chemical Stoichiometry Problems
In this stoichiometry worksheet, learners calculate theoretical yield and percent yield. This worksheet has 2 problems to solve.
Curated OER
How is a REDOX Reaction Used to Power a Car?
Young scholars explore electrolysis and Redox reactions. In this exploratory lesson students demonstrate an example of electrolysis and how it brings on a chemical change.
Curated OER
Anhydrides, Acid Rain and Titrations
In this chemical compounds worksheet, students determine is compounds will act as acids or bases when added to water. Students compare basic, acidic, and amphoteric anhydrides. This worksheet has 15 fill in the blank, 2 short answer...
Curated OER
Reaction Kinetics
In this chemical reactions worksheet, students determine the reaction rate for various reactions, write balanced equations, create graphs showing the reaction over time, and calculate the activation energy and potential energy. This...
Virginia Department of Education
Molar Volume of a Gas
What is a chemist's favorite plant? Stoichiome Tree! Scholars produce hydrogen gas by reacting magnesium with hydrochloric acid. Then they calculate the molar volume of the gas produced before answering assessment questions.
Virginia Department of Education
Solution Concentrations
What happens when you combine 6.022 times 10 to the 23 piles of dirt into one? You make a mountain out of a mole hill. Scholars use dehydration to obtain percent composition and then calculate the molarity of the original...
Virginia Department of Education
Partial Pressure
At some point, everyone has been under pressure—even Dalton! Explore Dalton's law of partial pressures with young chemists as they measure the volume of air extracted from a sample compared to its original volume. Class...
Virginia Department of Education
Soap, Slime, and Creative Chromatography
Do you think chromatography paper suffers from separation anxiety? Young chemists make soap, slime, silly putty, and experiment with chromatography in this lesson. The material includes clear instructions for each experiment along with...