CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] Based on the balanced chemical equation, students will calculate the masses or moles of reactants or products generated in a given reaction. They will also convert...
Carnegie Mellon University
Chem Collective: Stoichiometry Bridge Course
This is a complete course in chemical stoichiometry, set in a scenario that shows how stoichiometry calculations are used in real-world situations. This course has been designed to not only help you strengthen your skills with...
Clackamas Community College
Clackamas Community College: Decomposition Reactions
This chemistry tutorial describes decomposition reactions with two examples.
Khan Academy
Khan Academy: The Reaction Quotient Q
Definition of reaction quotient Q, and how it is used to predict the direction of reaction.
CK-12 Foundation
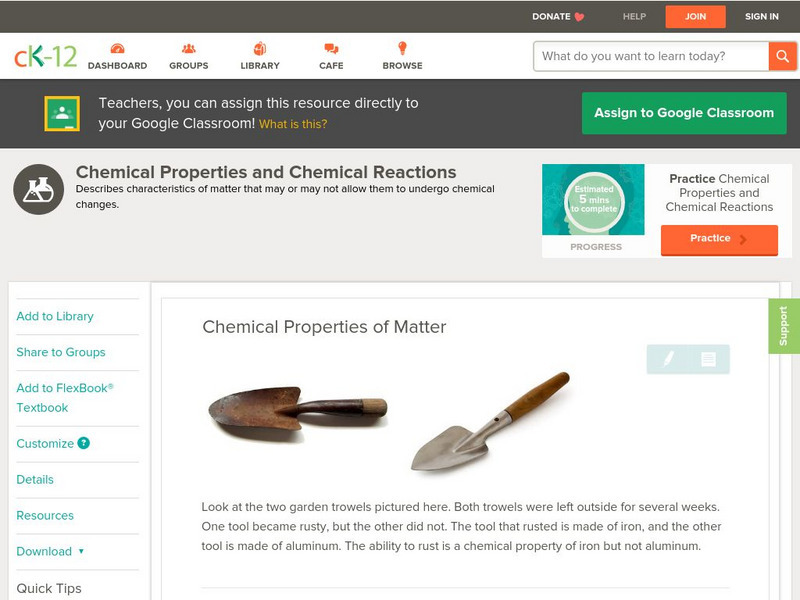
Ck 12: Physical Science: Chemical Properties of Matter
Definition of chemical property and examples of the chemical properties of matter. [Free Registration/Login may be required to access all resource tools.]
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Stoichiometry
This online textbook chapter provides learners with advanced-level reading and practice material on stoichiometry
Science Buddies
Science Buddies: Saturated Solutions: Measuring Solubility
Many essential chemical reactions and natural biochemical processes occur in liquid solutions, so understanding the chemical properties of liquid solutions is fundamentally important. This project will challenge you to discover how much...
Georgia Department of Education
Ga Virtual Learning: Chemistry: Stoichiometry
Through informational text, interactive practice problems, video clips, and real-world application, students are introduced to the science of Stoichiometry.
Science Buddies
Science Buddies: What Makes Ice Melt Fastest?
If you live in a place that gets cold in the winter, you have most likely seen trucks spreading a mixture of sand and salt on the streets after a snowfall to help de-ice roads. This basic chemistry project gives you clues to discover how...
Georgia Department of Education
Ga Virtual Learning: Chemistry: Thermochemistry
Through informational text, interactive practice problems, online labs, and virtual simulations, students are introduced to the concepts of thermochemistry.
PBS
Pbs Teachers: Scientific American: Beneath the Sea: Light Stick Chemistry
Explore chemiluminescence and describe how the temperature of the chemicals that combine in a light stick affects the reaction. Apply that knowledge to understand bioluminescence in deep-sea marine life.
Chem4kids
Chem4 Kids: Physical Chemistry Basics
Discover what physical chemistry is all about in this brief overview.
Nobel Media AB
The Nobel Prize: Chirality Chemistry 2001
In 2001 the Nobel prize in chemistry was awarded for work with Chiral molecules. These molecules can be used to control the speed of chemical reactions. This brief game teaches the basic principles of chirality.
California State University
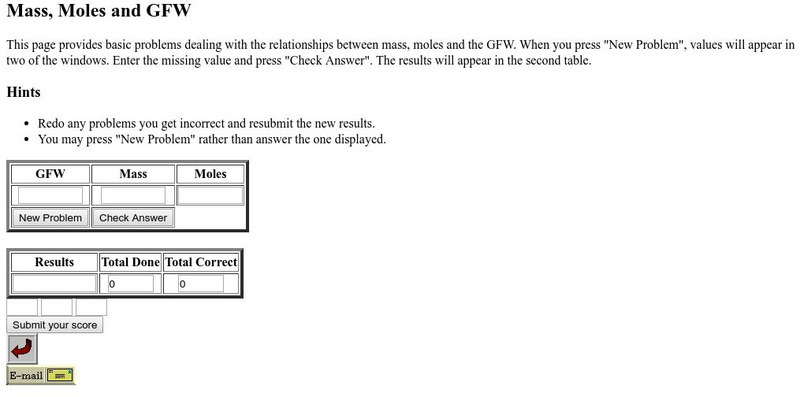
Csudh Project for Chemistry: Mass, Moles, and Gfw
Several basic problems dealing with the relationships between mass, moles, and the GFW.
Upper Canada District School Board
Tom Stretton's Advanced Placement Chemistry: Electrochemistry
This online textbook chapter provides learners with advanced-level material on electrochemistry.
CK-12 Foundation
Ck 12: Thermochemical Equations
[Free Registration/Login may be required to access all resource tools.] Students investigate the conditions under which the enthalpy change in a reaction is equal to the heat absorbed or released. Then, they have the opportunity to write...
Dartmouth College
Dartmouth College: Chem Lab: Coordination Chemistry 3.1: Acid/base Analysis
In this experiment, you will examine the acidity of your coordination complex's water ligand. You will determine the acid dissociation constant of the complex by titrating it with a base. There are eight weeks of experiments in this series.
CK-12 Foundation
Ck 12: Free Energy and Equilibrium
[Free Registration/Login may be required to access all resource tools.] Students determine the temperature at which a reversible reaction will achieve equilibrium by using the Gibbs free energy equation, and then describe the...
Frostburg State University
General Chemistry Online: Chemical Change Faq
Investigate the answers to many commonly asked questions about chemical change. This comprehensive list will address chemical equations and double displacement reactions in addition to other topics.
Other
Aspirin Foundation: The Chemistry of Aspirin
A simple overview of the chemical structure and function of aspirin. Follow links to learn about the story of aspirin, its reactions, and related products.
Khan Academy
Khan Academy: Stoichiometry
How to use mole ratios from a balanced reaction to calculate amounts of reactants.
CK-12 Foundation
Ck 12: Net Ionic Equations
[Free Registration/Login may be required to access all resource tools.] Students write net ionic equations for double-replacement reactions that produce precipitates, gases, or molecular compounds, write net ionic equations for...
TeachEngineering
Teach Engineering: If You're Not Part of the Solution!
Students continue the research begun in the associated lesson as if they were biomedical engineers working for a pharmaceutical company. Groups each perform a simple chemical reaction (to precipitate solid calcium out of solution) to...