Curated OER
A Solution for Precipitation
Students predict the product of chemical reaction using the solubility rules. In this chemistry lesson, students balance ionic equation. They perform a lab to check if their predicted products are correct.
Curated OER
Equilibrium Equations
In this equilibrium worksheet, students calculate the equilibrium constant for reactions and the equilibrium concentrations. This worksheet has 10 problems to solve.
Curated OER
Chapter 5 Practice Problems
In this chemistry worksheet, students determine the oxidizing agent and the reducing agent in each stated problem. Then they determine the molarity of acetic acid. Students also use the previous activities to predict whether a...
Curated OER
Chemical Reactions
In this chemical reactions worksheet, students determine if solutions are acidic, basic, or neutral. Students give reactions' molecular equations and ionic equations. This worksheet has 14 problems to solve.
Curated OER
Spontaneous Cell Reaction
In this spontaneous cell reaction worksheet, students write equations for these types of reactions and determine the voltage associated with the cell. This worksheet has 4 problems to solve.
Curated OER
Chemical Reactions
In this chemical reactions worksheet, students write chemical equations, draw and label isomers, and predict products of chemical reactions. This worksheet has 5 drawings, 1 short answer, and 7 fill in the blank questions.
Curated OER
Coordination Compounds
In this coordination compounds learning exercise, students determine the name, coordination sphere, center of coordination, ligands, and coordination number for given compounds. This learning exercise has 5 fill in the blank questions.
Curated OER
Chemical Reactions
In this science worksheet, learners use basic scientific concepts to complete the series of puzzles that are intended to increase science literacy and one activity has one name the type of chemical reaction that is taking place.
Curated OER
Water
In this water instructional activity, students compare and contrast elements and compounds. Students define chemical symbols, chemical formulas, and chemical properties. This instructional activity has 19 short answer questions.
Curated OER
Calculating Rates
In this rates worksheet, students determine the rate of production or consumption for given reactions. Students write chemical equations in complete ionic form or net-ionic form. This worksheet has 6 problems to solve.
Curated OER
My Test Book: Percents
In this online interactive math skills activity, students solve 10 multiple choice math problems regarding percents. Students may submit their answers to be scored.
Curated OER
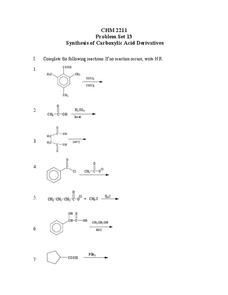
CHM 2211: Problem Set 13 Synthesis of Carboxylic Acid Derivatives
In this chemistry learning exercise, students work on a problem set consisting of 16 equations in which they synthesize carboxylic acid derivatives.
Curated OER
Hot Gas Or Cold Gas Lab
Students engage in a lab project to study chemical reactions. They use guided questions to help facilitate the lab experience and come to the correct outcomes. The lesson does not contain a true understandable objective. The lesson could...
Virginia Department of Education
Charles’ Law
Searching for a relatively interesting way to demonstrate Charles' Law? Here is a instructional activity in which pupils heat air inside a flask and then cool the flask to quickly cool the air. They make observations about what occurs...
Curated OER
Thermochemistry Calculations
In this chemistry worksheet, students apply reaction equation ratios to solve fifteen problems, including five using a specific formula provided on the worksheet.
Virginia Department of Education
Molar Volume of a Gas
What is a chemist's favorite plant? Stoichiome Tree! Scholars produce hydrogen gas by reacting magnesium with hydrochloric acid. Then they calculate the molar volume of the gas produced before answering assessment questions.
Virginia Department of Education
Solution Concentrations
What happens when you combine 6.022 times 10 to the 23 piles of dirt into one? You make a mountain out of a mole hill. Scholars use dehydration to obtain percent composition and then calculate the molarity of the original solution.
Virginia Department of Education
Partial Pressure
At some point, everyone has been under pressure—even Dalton! Explore Dalton's law of partial pressures with young chemists as they measure the volume of air extracted from a sample compared to its original volume. Class members perform...
Pingry School
Chemical Equilibrium
We know about the light spectrum, the age spectrum, and sound spectrum, but do chemical reactions also occur on a spectrum? Young scientists experiment with partial reactions on a spectrum and observe the color changes. Then, they...
Virginia Department of Education
Thermochemistry: Heat and Chemical Changes
What makes particles attract? Here, learners engage in multiple activities that fully describe colligative properties and allow the ability to critically assess the importance of these properties in daily life. Young chemists conduct...
Pingry School
Solubility Product of an Ionic Compound
How do scientists determine when a solution is fully saturated? Scholars address the topic as they observe patterns of precipitation in various concentrations of ions. Using a well plate, pipette, and common chemicals, they collect data...
Pingry School
Synthesis of an Insoluble Ionic Salt: A Stoichiometry Experiment
Challenge young scientists to design their own experimental procedures. They write the procedure for properly preparing two grams of a water-insoluble ionic salt. To finish, they perform the experiment and collect data to prove their...
Curated OER
Percent Yield
In this percent yield worksheet, students solve 4 problems. They find the percent yield given the chemical reaction, the mass of the reactants and the mass of the products. Given the percent yield students find the liters of a substance...
Virginia Department of Education
Finding the Formula and Percent Composition
Do you have mole problems? If so, call Avogadro at 602-2140. The lesson plan starts with pupils working independently to solve for molar mass of ionic compounds. Then they learn to solve for percent composition and later perform an...