Curated OER
Lesson #2 ~ Nanoscience and Nanotechnology
You might love this lesson, or you might not. Basically, high school scientists read through a script in which someone interviews a physicist, a biologist, and a chemist in regard to their use of nanotechnology. The names of the involved...
Curated OER
Solids, Liquids, and Gases
Not many lessons on states of matter can hold a candle to this one! Junior chemists gauge the density of paraffin in both liquid and solid phases. They fill 60 mL syringes with different materials to experiment with compressibility and...
Curated OER
Dilution and Concentration of Solutions
Future chemists practice laboratory techniques by creating a monochloramine solution. The objectives are to use of dilution, 9concentration, and measurement skills and to prepare a solution that will be used in a water treatment...
Curated OER
Gasoline Additive
Chemists consider a situation in which an ethanol producer needs to determine how much to add to t-butanol to prevent freezing during transport. They work in the laboratory to obtain the freezing point depression constant for the...
Curated OER
Determination of Phosphorus Content in River Water
Divide your chemistry or environmental science class into two groups. Each group tests water samples from a river for the concentration of phosphorus using a different method. With chemists, you can use this activity as they learn to use...
Curated OER
Chalk Fizz
Little chemists observe the effects of acid on calcium carbonate as an example of chemical change. As a demonstration you will place a raw egg in vinegar overnight, and as a lab activity, learners drip vinegar onto a piece of chalk....
Curated OER
Acid Rain Effects
Get out the goggles and conduct a simple experiment to model and explore the harmful effects of acid rain (vinegar) on living (green leaf and eggshell) and non-living (paper clip) objects. Young chemists observe and describe the harmful...
Curated OER
Cosmic Chemistry: An Elemental Question
Space scientists investigate the cosmic phenomena in order to search for answers to possible origins of the solar system. They consider the concepts of elements and isotopes. Data is analyzed looking at tje abundance of elements...
Curated OER
Avoiding Carbon Dioxide Emissions from Burning Fossil Fuels
Chemistry and earth science meet in a lesson on carbon dioxide emissions. After reading about atmospheric problems caused by using fossil fuels, science stars balance equations for the burning of different alkanes. They compute the...
Curated OER
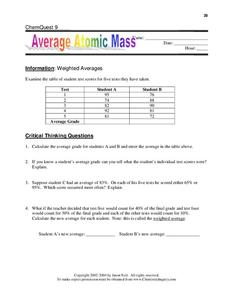
Average Atomic Mass
Using a chart of student test grades as an example, curious chemists learn how to calculate weighted averages. They apply this knowledge to elements on the periodic table. As practice in calculating average atomic masses, learners...
Curated OER
Light Stick Chemistry
In groups of three with the lights off and the shades drawn, investigators place inactivated light sticks, in three beakers: one filled with ice water, another with lukewarm water, and the other with room temperature water. They wait...
Curated OER
Heat and Thermodynamics
This is actually a 10-day mini unit on thermal energy for your high school chemists. Every avenue is taken to get learners absorbed in heat: a pretest, a PowerPoint presentation, Internet exploration, demonstrations, lab activities, and...
Curated OER
Ionic Nomenclature
One document contains five different worksheets for practice naming and writing formulas for ionic compounds. The first is particularly notable, as it systematically walks beginning chemists through the process of using the periodic...
Lesson Snips
Who Killed the Flowers?
This could be really good, or it could be really bad! The crime to be solved is, "Who went pee in the flowerpot?" Given four imitation urine samples, young chemists or crime scene investigators perform pH, glucose, and turbidity...
Curated OER
2004 U.S. National Chemistry Olympiad Exam Part II
In this National Chemistry Olympiad test, junior chemists answer eight problem solving questions on a variety of topics. These include calculating molarity of solutions, determining rates of reactions, calculating decay and discussing...
Curated OER
Chemistry 231, Exam 3
This exam is aimed at assessing college level organic chemists. Ten problems are to be solved and include drawing molecular structures, assessing degrees of saturation, ordering alkenes according to stability, and explaining chemical...
Curated OER
Periodic Table Basics Test
The periodic table of elements is a vital tool for all chemistry apprentices and professionals alike. Here is a chance for your beginning chemists to assess their understanding of the periodic table. They list element names, symbols,...
Curated OER
Laboratory: Micro Rockets
If you know how to employ the exothermic reaction between hydrogen gas and oxygen gas to make a miniature rocket, then this worksheet is a fabulous lab sheet for your chemistry charges. First, they observe a spark in pure oxygen and one...
Curated OER
Laboratory: Fe and Cu (II) Redox Reaction
In this redox reaction laboratory sheet, chemists answer eight post-lab questions about oxidizing agents, reducing agents, oxidized and reduced substances, and percent yield of products. You will need to provide a proedure for...
Curated OER
WS 4.6 Types of Reactions
Somehow 55 chemistry problems have been squished onto a single page! Don't assume that it is a mess, however. High school chemists write the products for different types of reactions. Then they identify the type of reaction in addition...
Curated OER
Electron Dot Structures, etc.
In this electron dot structures handout, new chemists read about electron arrangement and how to draw electron dot diagrams. They complete a chart with the mass number, atomic number, isotope notation, number of neutrons, and Bohr...
Curated OER
Bond Type
At the top of the page are a reading passage and colorful diagram that depicts the tug-of-war that occurs between bonding molecules due to electronegativity. High school chemists fill in a chart with electronegativity values, the...
Curated OER
The Atom Board - Making Atoms
In this atom worksheet, chemists learn how to determine the number of protons, neutrons and electrons in an atom using the atomic mass and atomic number. They complete a chart of the subatomic particles and use marbles to represent...
Curated OER
Periodic Table Notes: Determining # of Shells and Valence Electrons
Although the directions say to view a PowerPoint, it is not necessary in order to complete this assignment. Six fill in the blank questions survey the periodic table of elements. A chart follows in which, given the element symbol, young...