Simon Fraser University
Chem1 Virtual Textbook: The Quantum Numbers
Acting as a subtopic of the General Chemistry Virtual Textbook's section on Atoms and the Periodic Table, this site discusses quantum numbers of electrons in atoms including topics such as principal quantum number and orbitals of the...
University of Colorado
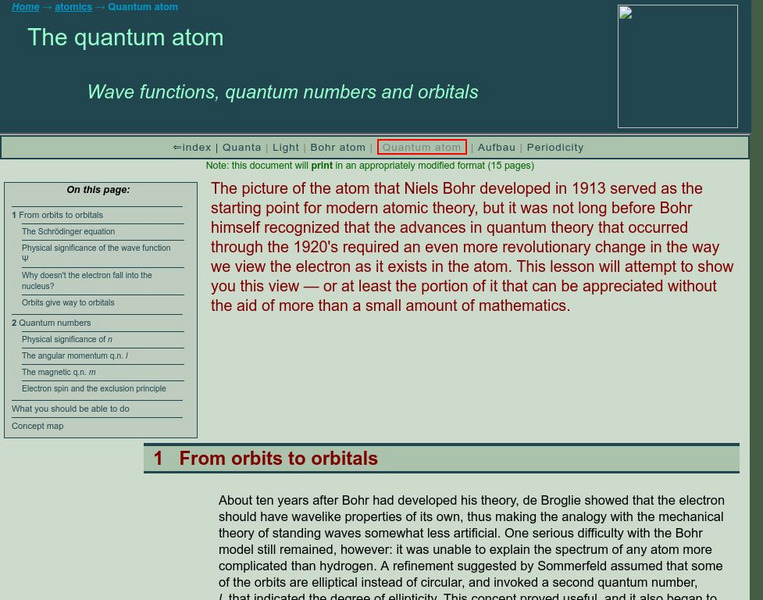
University of Colorado: Physics 2000: Bohr's Atom
An outstanding site that can best be described as student friendly. It describes the main differences between the classical and quantum models of the atom. Clear and well illustrated.
Friesian School
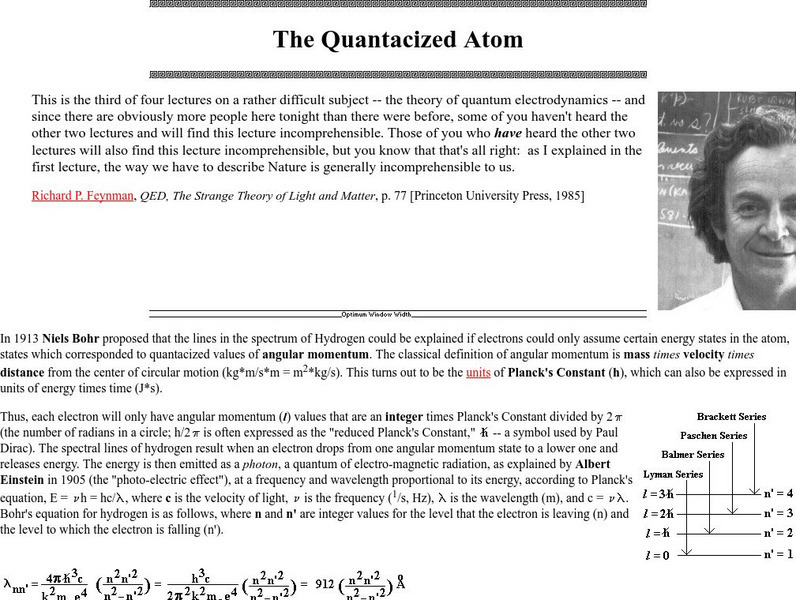
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Science Kids: Science Images: Hydrogen Atom
This is a simple picture of a hydrogen atom using the Bohr model. A negatively charged electron can be seen on the outside of the positively charged proton.
State University of New York
State University of New York: Atomic Absorption and Emission
This module simulates the excitation of hydrogen atoms through irradiation with electromagnetic radiation of different wavelengths.
Concord Consortium
Concord Consortium: Reaction Between Hydrogen and Oxygen Atoms
Explore energy during a reaction between hydrogen and oxygen atoms.
Other
Atom Central: The H Bomb & the Birth of the Universe
Peer into the heart of nuclear explosions through a series of photographs of the detonation of Hydrogen Bombs.
Ducksters
Ducksters: Chemistry for Kids: Elements: Hydrogen
On this website, the element hydrogen and its chemistry including atomic weight, atom, uses, sources, name, and discovery are discussed. Plus properties and characteristics of hydrogen.
CK-12 Foundation
Ck 12: The Bohr and Quantum Models of the Atom
[Free Registration/Login may be required to access all resource tools.] Students explore how the study of the hydrogen emission spectrum led to the Bohr model of the atom, in which electrons exist in states of constant energy.
Curated OER
Brockport High School: Energy Levels of Hydrogen Atom
From the Brockport High School Physics Labs web pages. Includes an excellent graphic depicting the energy levels of a hydrogen atoms and portraying the electron level transitions for the Lyman, Balmer, and Paschen series. Includes both...
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 2
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 2 of 4 in the series titled "Molecular Structure of Water."
Sophia Learning
Sophia: Molecular Structure of Water: Lesson 3
This lesson will introduce the molecular structure of water, including the Hydrogen atoms, Oxygen atoms, and types of bonds. It is 3 of 4 in the series titled "Molecular Structure of Water."
Walter Fendt
Walter Fendt: Apps Zur Physik
This site, in German, offers numerous apps that illustrate common physics principles. Apps are organized into categories: mechanics, oscillations and waves, electrodynamics, optics, thermodynamics, the theory of relativity, physics of...
University Corporation for Atmospheric Research
Ucar: Hydrocarbons
Learn about "hydrocarbons", chemical compounds whose molecules are made up entirely of carbon and hydrogen atoms.
TED Talks
Ted: Ted Ed: How Polarity Makes Water Behave Strangely
Water is both essential and unique. Many of its particular qualities stem from the fact that it consists of two hydrogen atoms and one oxygen, therefore creating an unequal sharing of electrons. From fish in frozen lakes to ice floating...
Other
Atom Central: The Cuban Missile Crisis
A timeline of events between October 15 and November 1, 1963 depicts the time period during which the Cuban Missile Crisis took place,
Georgia State University
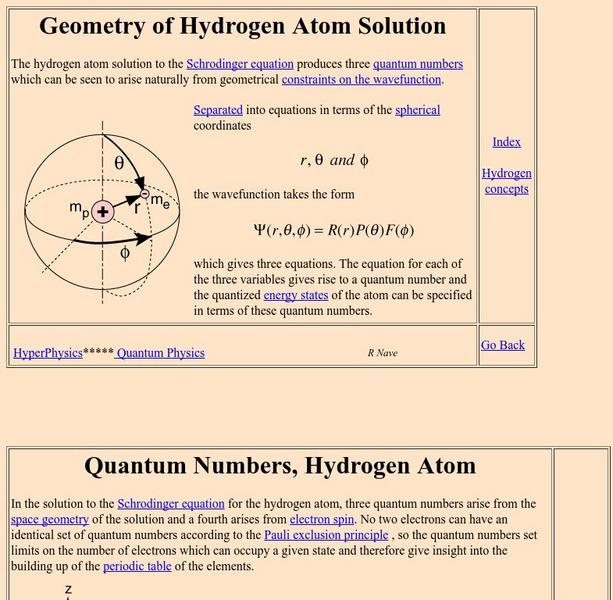
Georgia State University: Hyper Physics: Quantum Numbers, Hydrogen Atom
This tutorial contains links to explanations of the four different quantum numbers (principal, orbital, magnetic, and spin). Equations for each are provided.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Chemistry 1934
At this website from The Nobel e-Museum, read about Harold Clayton Urey (1893-1981 CE), the chemist awarded with a Nobel Prize "for his discovery of heavy hydrogen." Download Urey's Nobel Lecture, "Some thermodynamic properties of...
CK-12 Foundation
Ck 12: Polarity and Intermolecular Forces
[Free Registration/Login may be required to access all resource tools.] The following online tutorial describes how the electronegativity difference between two atoms in a covalent bond results in the formation of a nonpolar covalent,...
Georgia Department of Education
Ga Virtual Learning: Ap Biology: Chemistry of Life
Through informational text, interactive activities, animations, and video clips, students examine the chemistry of living things, and they learn how interactions from atoms are fundamental to life as we know it.
OpenStax
Open Stax: Chemical Bonds
Learn here about chemical bonds, a weak or strong electrical attraction that holds atoms in the same vicinity.
University of St. Andrews (UK)
University of St. Andrews: Niels Henrik David Bohr
This resource presents a biography of Bohr, including many personal tidbits, as well as a substantial treatment of his scientific work. Several interesting quotes by and about Bohr are included. It also links to several other sources.
NASA
Nasa: Space Place: Play Helios: A Game About How the Sun Makes Energy!
In this pairing game, keep the Sun shining bright by matching up particles. Convert hydrogen atoms into helium atoms. This process is called nuclear fusion.
Encyclopedia of Earth
Encyclopedia of Earth: Solar Radiation
Almost all of the energy that drives the various systems (climate systems, ecosystems, hydrologic systems, etc.) found on the Earth originates from the sun. Solar energy is created at the core of the sun when hydrogen atoms are fused...