Curated OER
Empirical Formulas
Students investigate empirical formulas for molecules. In this empirical formulas lesson plan, students use paper ions to form formulas and they show these are empirical formulas. They use ions of carbon and hydrogen showing electron...
Curated OER
Chemical Bonding and Shapes of Molecules
In this chemical bonds worksheet, students review the different types of bonds, Lewis dot structures, ions, and molecule shapes. This worksheet has 10 matching, 17 multiple choice, and 3 drawing questions.
Curated OER
Chemical Compounds
In this chemistry learning exercise, students identify 3 different chemical compounds, 3 identify biochemical products, 4 identify terms, and 2 identify atomic symbols.
Curated OER
Pre-Lab Questions-Acids and Bases
In this acids and bases instructional activity, students answer eight questions about acids and bases prior to doing a titration lab. They define both acids and bases and explain what a neutralization reaction is as well as a titration.
Curated OER
Solubility and Intermolecular Forces
In this solubility and intermolecular force activity, students are given 26 clues about forces between molecules such as hydrogen bonds and London forces and about solubility of solutions.
Curated OER
Acids and Bases
In this acid and base activity, students are given terms related to acids and bases and their definitions. Students match the proper definition to the terms but cutting out the definitions and gluing them next to the proper term.
Curated OER
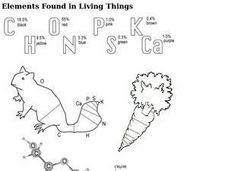
Elements Found in Living Things
In this elements learning exercise, students review the most common elements found in living things. Students color in two pictures with the percentages of the elements found in that living thing.
Curated OER
Acid, Base, or Salt?
In this acids and bases worksheet, high schoolers read about the differences between acids and bases and then complete a table comparing the characteristics of both. Then students determine if the given statements describe an acid, base,...
Curated OER
Effect of Concentration of the Reactants
Students experiment to determine the effect of the frequency of collisions between atoms or ions of the reactants. They investigate the rate at which aluminum replaces hydrogen from a solution of hydrochloric acid.
California Academy of Science
Coral and Chemistry
Using cabbage juice as a pH indicator, future scientists explore the effect of increasing carbon dioxide on the pH of the ocean and relate it to the health of coral reefs. Ideal for an earth or environmental sciences course, this...
LABScI
Acids and Bases: Cabbage Juice pH Indicator
Explore the range of pH using an assortment of household liquids. Scholars create their own pH indicators from cabbage and determine the pH of several liquids. To further their exploration, individuals use the same liquids to create...
Chymist
Visualizing pH
Why are acids and bases important in our daily lives? Lead the class in answering this question, among others, as they experiment with pH paper and classify where various substances belong on the pH scale. They also taste common acids...
Science Geek
Properties of Acids and Bases
It's all about that base! Teach the properties of acids and bases using the sixth slide show presentation in a series of seven. The lesson discusses acid and base properties and reactions. Pupils also see the effect on indicators when...
Curated OER
Chapter 14 Review, Section 2: Acids and Bases
Lewis acids and bases, Brønsted-Lowry acids and bases, ionization, and more are covered by this chemistry handout. It serves as a review of a specific textbook section, but will also serve as a nice review for any general chemistry...
Curated OER
Determination of An Acid Dissociation
In this chemistry worksheet, students examine the given concept in order to apply in the laboratory setting. The sheet includes in depth background information.
Virginia Department of Education
Acids and Bases
What did one titration say to the other titration? We should meet at the end point! Young chemists perform four experiments: dilute solution, neutralization, titration, and figuring pH/pOH.
Virginia Department of Education
Acid-Base Theory
Litmus paper, why so blue? A chemistry lesson plan includes a pre-lab activity, practice calculating pH, an experiment measuring the pH in acids and bases, a titration demonstration, and a titration experiment.
NASA
A Different Perspective
What can we learn from the data? Young scholars analyze actual solar data to answer specific questions. The activity presents an opportunity for an open-ended investigation of the data to conclude a five-part series on solar winds.
NASA
Christa's Lost Lesson: Effervescence
How are chemical reactions affected by gravity? Learners explore the phenomenon of effervescence as part of the Christa's Lost Lessons series. They compare findings in an experiment on effervescence to a video of a similar experiment in...
Curated OER
Photosynethsis....How do we know it's really happening? - Biology Teaching Thesis
High schoolers think of one way to detect photosynthesis in plants besides those ways shown in class. They identify the reaction that occurs during photosynthesis. Students define the difference between an acid and a base. They identify...
Curated OER
Dissolved Oxygen and Respiration
Students are presented with the question, "Do plants that grow underwater use oxygen?" They create an experiment to test the presence of dissolved oxygen in the water using provided materials. Student experiments include a control jar as...
Curated OER
Tides in the Hudson
Students view an illustration of the Hudson River watershed and identify the bodies of water shown. They discuss what happens when fresh and salt water mix. Students view a teacher demonstration of the stratification of fresh and salt...
Curated OER
Change Since 1609
Students recognize how the climate of the Hudson Valley has changed since the last glaciation. They explain these changes using a reconstruction of the land use changes in the Hudson Valley composed of confetti, Ziploc bags and other...
Curated OER
Paleoclimate of the Hudson Valley
Learners recognize how the climate of the Hudson Valley has changed since the last glaciation and be able to explain these changes. They reconstruct the paleoclimate of the Hudson Valley.