Curated OER
Chapter 14 Review, Section 1: Acids and Bases
An overview of acid and base compounds and their reactions is presented with this handout. Pupils name compounds and write chemical formulas. They write net ionic equations and explain how acidic solutions might conduct electricity. This...
Curated OER
Unit 3 Bonding
An organized table charting the different types of chemical bonds arrays this resource. The octet rule, ionization energy, and the naming of compounds are also reviewed. Young chemists answer review questions in multiple choice fashion....
Curated OER
Chemistry 1 Practice Exam
Thirty multiple-choice questions and their answers are provided in this resource. It was written for a general chemistry course and queries test takers on scientific notation, mixtures, chemical symbols, electric charges, Dalton's atomic...
Curated OER
Atoms, Ions and Formula Basics Make-up Test
Using a copy of the periodic table, chemistry test takers fill in a chart with element name, chemical symbol, atomic number, atomic mass, and numbers of subatomic particles. They define subatomic particles, draw atom models, explain...
Museum of Science
goREACT
Beginning Boyles and curious Curies can safely experiment with the virtual mixing of elements using this entertaining application.
It's About Time
How Atoms Interact with Each Other
Connect the dots and assist young chemists as they demonstrate covalent and ionic bonding. Class members use their knowledge of valence electrons to predict compound formulas as they arrange electrons into various bonding structures to...
Curated OER
Chemistry Foundations
Extensive notes on foundational chemistry concepts make up this resource. It summarizes the properties of matter, the periodic table, chemical nomenclature, and general chemical bonding. Design a set reading comprehension questions to go...
Pingry School
Synthesis of an Insoluble Ionic Salt: A Stoichiometry Experiment
Challenge young scientists to design their own experimental procedures. They write the procedure for properly preparing two grams of a water-insoluble ionic salt. To finish, they perform the experiment and collect data to prove their...
Curated OER
Ionic Compounds and Writing Formulas
In this ionic compounds worksheet, high schoolers write the chemical formulas and names of 28 ionic compounds to complete the chart.
Curated OER
Naming Compounds
In this compounds worksheet, students compare ionic, covalent, and polyatomic compounds and give examples of each type. This worksheet has 1 matching, 3 problem solving, and 4 short answer questions.
Santa Monica College
Single and Double Displacement Reactions
If you aren't part of the solution, you are part of the precipitate! Young chemists learn about single and double displacement reactions including precipitation reactions, neutralization reactions, and gas forming reactions. They perform...
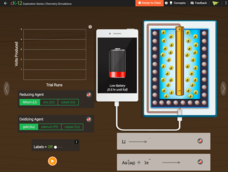
CK-12 Foundation
Battery
Don't take for granted the technology behind power packs. Build an understanding of the chemical mechanics of a battery pack that charges your phone. The simulation allows young scientists to manipulate the type of elements in a pack and...
Curated OER
Predicting and Naming Polyatomic Ionic Compounds Worksheet
In this compounds worksheet, learners write the polyatomic ionic compound formulas or the compound names. This worksheet has 29 problems to solve.
Curated OER
Mixed Ionic Compounds
In this compounds worksheet, students write the formulas for the given ionic compounds and write the names for the ionic compounds shown. This worksheet has 20 fill in the blank questions.
Curated OER
Chemical Bonding and Shapes of Molecules
In this chemical bonding worksheet, students review the different types of bonds and calculate the number of valence electrons in molecules. This worksheet has 11 matching and 15 multiple choice questions.
Curated OER
Compound Names and Formulas
In this compounds worksheet, students name the given ionic compound, describe how covalent compounds are named, and write the chemical formulas for the covalent compounds. This worksheet has 2 fill in the blank and 3 short answer questions.
Curated OER
Is it Ionic?
In this ionic compounds worksheet, students are given clues about compounds such as their physical and chemical properties in order to determine if they are ionic compounds. Students indicate which compounds are ionic and which are not.
Curated OER
Differentiate Elements, Compounds, and Mixtures
Young scholars examine the differences between elements, compounds and mixtures. Using diagrams, they compare and contrast atoms and molecules and describe various chemical reactions. They distinguish the differences between ionic and...
Curated OER
Ionic and Covalent Bonds
Ionic and covalent bonds are the focus of this chemistry activity, which provides learners with eighteen key terms to use in a fill-in-the-blank activity. Additionally, students are prompted to write the number of atoms in four given...
Curated OER
Chemical Analysis and Stoichiometry
In this chemical analysis worksheet, students use a combustion analysis report to calculate the empirical formula for compound samples. This worksheet has 2 problems to solve.
Curated OER
Learning Outcomes
In this science worksheet, students explore the learning outcomes for a unit on chemical reactions. Students define 60 vocabulary words and answer a list of questions for each topic.
Curated OER
Worksheet 7 - A Practice Quiz 3
Every sort of chemical bond is touched upon during this assignment. Chemistry whizzes identify what type of bond is formed by analyzing chemical formulas or Lewis structure diagrams. Multiple choice questions also ask learners about...
Curated OER
Chapter 13 Review, Section 1: Ions in Aqueous Solutions and Colligative Properties
This is an apt assignment for chemistry takers that are studying ionic solutions. Eight questions require problem solving and critical thinking to answer. The first question instructs learners to use a table in the textbook, but you can...
Curated OER
What Happens When Chemicals Are Mixed Together?
In this naming acids worksheet, students are given names of anions and they write the anion symbols. Students then write the formula and name of each acid.