Creative Chemistry
Preparation of Crystalline Derivatives of Aldehydes and Ketones
Chemistry explorers prepare a crystalline derivative and find its melting point. Once they discover the melting point, they can identify whether the substance is pure or an aldehyde or ketone. This outstanding laboratory activity helps...
Curated OER
Physical Properties of Matter
Five fabulous procedures introduce physics or chemistry classes to special properties of matter. They discover adhesion and cohesion, solubility, melting and boiling points, and viscosity through hands-on experiences. Tests are...
Curated OER
Grossmont College - Chem 116 Practice Exam 3
This biochemistry practice exam queries takers on the organic molecules. Pupils write IUPAC names, identify boiling and melting points of different compounds, and more. This is definitely designed for a college-level biochemistry course,...
Curated OER
Properties of Matter
This PowerPoint is a gem! Seventy-eight slides present an entire introduction to matter in bullet-point fashion. Viewers learn about everything from mass and inertia to phase changes and gas laws! The only glitch is that the links to...
Pingry School
Effect of Solutes on Boiling Point
Anyone that lives around snow knows that adding salts to water increases its melting point. Are there solutes that affect the boiling point as well? A scientific experiment has learners add different solutes to water and then...
Curated OER
Energy and States of Matter
Characteristics of each state of matter are listed, the formulas for heating water are detailed, and values for boiling and heat of vaporization are presented. This simple slide show provides direct instruction as well as practice...
Curated OER
Preparation of Triiodomethane (iodoform)
In this preparation of triiodomethane instructional activity, learners use propanone to prepare triiodomethane. Students use filtration and recrystallization in order to make the product. They determine the melting point...
Curated OER
Phase Changes of Water
A micro-unit on the phase changes of water includes three laboratory activities. Junior scientists compare the densities of ice and water, and then they do the same for cold and warm water. They examine freezing and boiling temperatures....
Curated OER
Freezing and Boiling Point
In this freezing and boiling point graph worksheet, learners use the graph illustrated to respond to several questions that follow. They identify what is the freezing, boiling, and melting point of the substance in the graph. Students...
Curated OER
2000 U.S. National Chemistry Olympiad National Exam - Part I
The National Chemistry Olympiad exams are comprehensive tests covering an entire year of chemistry concepts. You can use them as practice for competing in the challenge, or simply as a review, or as an actual final exam for your...
Curated OER
Chapter 6 - Bonds
Although there are only 16 questions here, this chemistry handout makes a terrific unit assessment. It queries youngsters on the properties of ionic and covalent compounds, relates bond length tho stability and enrgy, compares polar and...
Concord Consortium
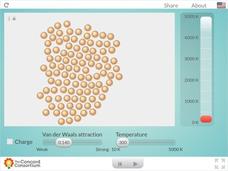
Charged and Neutral Atoms
Do charged and neutral particles behave differently as they undergo phase changes? Science sleuths examine two types of attractive forces using an informative interactive. Pupils can vary the amount of Van der Waals attraction present...
Curated OER
Physical and Chemical Trends in the Group 7 Elements
In this elements worksheet, students complete a graphic organizer by comparing the melting point, boiling point, density, and electronic configuration of given elements. Students determine the characteristics of Group 7 elements. This...
Curated OER
Physical Properties of Group 1 Elements
In this elements worksheet, learners complete a graphic organizer by filling in the symbol and atomic number for given elements. Students plot a graph of melting point against atomic number. Learners write the electron arrangements for...
Curated OER
11 - The Heat Is On
Students observe physical change of melting by observing substance in original state, melting substance, allowing substance to return to original temperature, determining if substance retained its original properties, and recording results.
Curated OER
Energy and Enthalpy
In this energy instructional activity, students calculate the specific heat and the melting point of titanium. Students calculate the energy released and compare the energetics for given reactions. This instructional activity has 4 word...
Curated OER
Chapter 13 Review, Section 2: Ions in Aqueous Solutions and Colligative Properties
Here is a general chemistry worksheet that incorporates practical applications. Four questions employ critical thinking about solutions, boiling and freezing points, and molar mass. Your class will practice what they learn in class by...
Curated OER
Chapter 13 Review, Mixed Review: Ions in Aqueous Solutions and Colligative Properties
Here is an attractive worksheet that walks chemistry learners through a review of aqueous solutions. There are matching, short answer, and multiple choice questions dealing with boiling and freezing points, precipitate, molality, net...
Messenger Education
Design Challenge: How to Keep Items Cool in Boiling Water
Keeping items cool in boiling water... what? This engaging activity challenges high school learners to build a container that keeps butter in a solid state when placing the container in boiling water. Groups use previous knowledge and...
Curated OER
States of Matter Summary
In this states of matter worksheet, learners are given a summary of the particle arrangement, the motion of particles, the properties of particles and models of particles in solids, liquids, and gases. Melting, evaporating and boiling,...
Curated OER
Temperature Change and the States of Matter
Tenth graders observe the processes of evaporation, condensation, melting, freezing, boiling, and sublimation. They do a quantitative investigation of the freezing of water, to explore explanations that involve particles.
Curated OER
Characteristics of Crystals
In this crystals worksheet, students complete a graphic organizer by filling in the characteristics of the different crystal types including melting/boiling point and electrical conductivity.
Royal Society of Chemistry
Symbols
Chemistry calculations can look a bit like alphabet soup at times. How do you help pupils make sense of it all? An interactive resource helps scholars sort through the symbols for common quantities such as moles, boiling point, and...
Curated OER
Ionic Compounds
For this compounds worksheet, students review the structure and properties of ionic compounds, their formulas and nomenclature, and molecular mass and percentage composition. This worksheet has 4 short answer questions and 7 problems to...