Curated OER
How a Seed Grows: And Who Grows It
Second graders explore botany by viewing video clips in class. For this seed growth lesson, 2nd graders identify the types of seeds that grow specific plants and what the optimal conditions are for growing seeds. Students view a video...
Curated OER
Effects of pH on Organic Molecules
Learners investigate the effects of an acid and a base on the structure of milk protein. They observe the changes to droplets of milk when adding ammonia and lemon juice and relate the changes to old, curdled milk. An extension using...
Curated OER
Martian Mummies
Students participate in "Raiders of the Future", a role play about futuristic scientists sending a spaceship to Mars for research and exploration. They collect geological samples for analysis, discover the ruins of an ancient...
Curated OER
How Soft or Hard is Your Water?
Students test samples of water to determine how a chemical water softener affects water's ability to form suds. After collecting their data and analyzing their results, students answer follow-up questions about their lab.
Libre Text
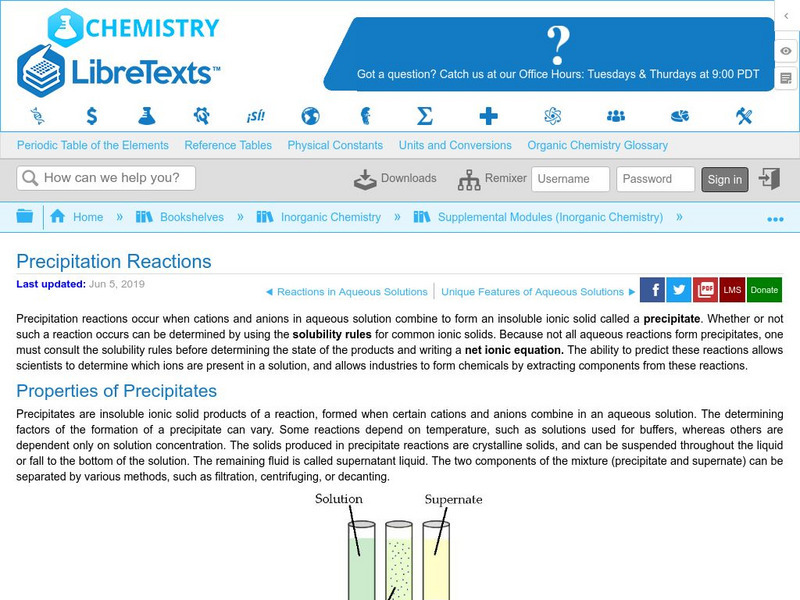
Libre Text: Precipitation Reactions
Precipitation Reactions occur when cations and anions of aqueous solutions combine to form an insoluble ionic solid, called a precipitate. Whether or not such a reaction occurs can be determined by using the solubility rules for common...
Other
Thermodynamics and Precipitation Reactions: Entropy and Precipitation
A colorful presentation of precipitation reactions with many diagrams and explanations. Very high level.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Solubility of Ionic Compounds & Precipitation Reactions
This interactive tutorial can teach you everything you need to know about solutions and precipitation reactions. Find out the solubility of specific ionic compounds and see how certain compounds can be insoluble in water.
Texas Education Agency
Texas Gateway: Precipitation Reactions
Given graphs, scenarios, illustrations, or descriptions, the student will determine how different processes affect solubility in aqueous solutions.
Georgia State University
Georgia State University: Hyper Physics: Precipitation Reactions
This site from Georgia State University discusses the precipitation reactions and deals with the lead iodide product. Links are provided to information on double replacement reactions as well as ionic compounds. Not too in-depth, this is...
CK-12 Foundation
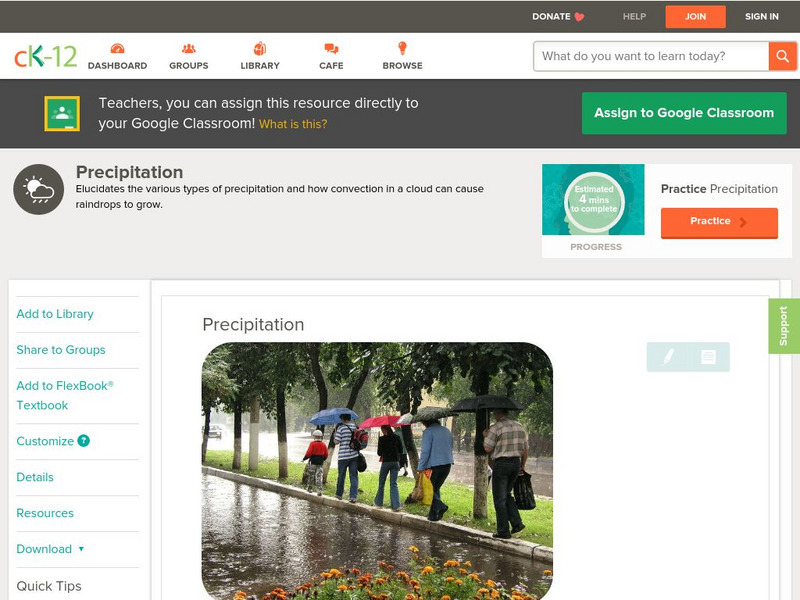
Ck 12: Earth Science: Precipitation
[Free Registration/Login may be required to access all resource tools.] Describes various types of precipitation.
CK-12 Foundation
Ck 12: Earth Science: Precipitation
[Free Registration/Login may be required to access all resource tools.] Describes various types of precipitation.
CK-12 Foundation
Ck 12: Earth Science: Precipitation
[Free Registration/Login may be required to access all resource tools.] Describes various types of precipitation.
CK-12 Foundation
Ck 12: Physical Science: Synthesis Reactions
[Free Registration/Login may be required to access all resource tools.] Definition and example of a synthesis reaction.
John Wiley & Sons
Wiley Higher Education Precipitation Reactions
This page has a RealVideo movie of the precipitation of mercury(I) iodide, with explanatory text.
John Wiley & Sons
Wiley Higher Education: Precipitation Reactions
This page has net ionic equations and animated graphics of reacting particles explain the precipitation of silver iodide.
American Chemical Society
Inquiry in Action: Formation of a Precipitate
A lab activity where students observe a chemical reaction by creating a precipitate. In this lab, students will create soap scum by combining hard water with soap. Lab activity includes both student and teacher information sheets.
Savvas Learning
Chemistry: The Central Science/metathesis Reactions
Discussion of metathesis (double replacement) reactions.
Khan Academy
Khan Academy: Double Replacement Reactions
Definition and examples of double replacement reactions. Predicting and balancing neutralization and precipitation reactions.
CK-12 Foundation
Ck 12: The P H Concept
[Free Registration/Login may be required to access all resource tools.] In this module, students will define pH and use the hydrogen or hydroxide ion concentrations to calculate the pH of a solution. They will also differentiate among...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Precipitation
An interactive animation depicting how a precipitate forms during chemical reactions. See how certain molecules attract and others repel when the particles start colliding.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: An Introduction to Chemical Reactions [Pdf] [Pdf]
A detailed introduction to chemical reactions, organized into two sections: "Chemical Reactions and Chemical Equations" and "Solubility of Ionic Compounds and Precipitation Reactions." Complete with examples, charts, and equations, this...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Writing Precipitation Equations
Use this step-by-step guide to determine whether solutions will form a precipitate and to write the equation.
Frostburg State University
General Chemistry Online: Reactions in Solution
Resource provides a set of 18 slides that discuss chemical reactions in solution. Slides 15-18 discuss metathesis (double replacement) reactions, including formation of insoluble precipitate.
Crescent Public Schools
The Internet Science Room: Types of Chemical Reactions
Students learn about the five different types of chemical reactions, as well as the three classifications of reactions.