American Chemical Society
Middle School Chemistry: Lesson Plans: Forming a Precipitate
Students investigate the concept of a precipitate by combining two clear, colorless solutions that produce a solid.
Khan Academy
Khan Academy: Gravimetric Analysis and Precipitation Gravimetry
Definition of precipitation gravimetry, and an example of using precipitation gravimetry to determine the purity of a mixture containing two salts.
American Chemical Society
Middle School Chemistry: Forming a Precipitate
This diagram illustrates a chemical reaction forming a precipitate.
CK-12 Foundation
Ck 12: Net Ionic Equations
[Free Registration/Login may be required to access all resource tools.] Students write net ionic equations for double-replacement reactions that produce precipitates, gases, or molecular compounds, write net ionic equations for...
CK-12 Foundation
Ck 12: Changes in Matter
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students identify the chemical properties of a substance and describe chemical changes and differentiate them from physical changes....
BBC
Bbc: Gcse Bitesize: Calculations for All Students
Law of conservation of mass: No atoms are created or destroyed in a chemical reaction. They just join together in a different way than they were before the reaction, and form products. This means that the total mass of the products in a...
CK-12 Foundation
Ck 12: Acid Base Neutralization
[Free Registration/Login may be required to access all resource tools.] Students will understand and differentiate among acid-base reactions, precipitation reactions, and oxidation-reduction reactions.
TeachEngineering
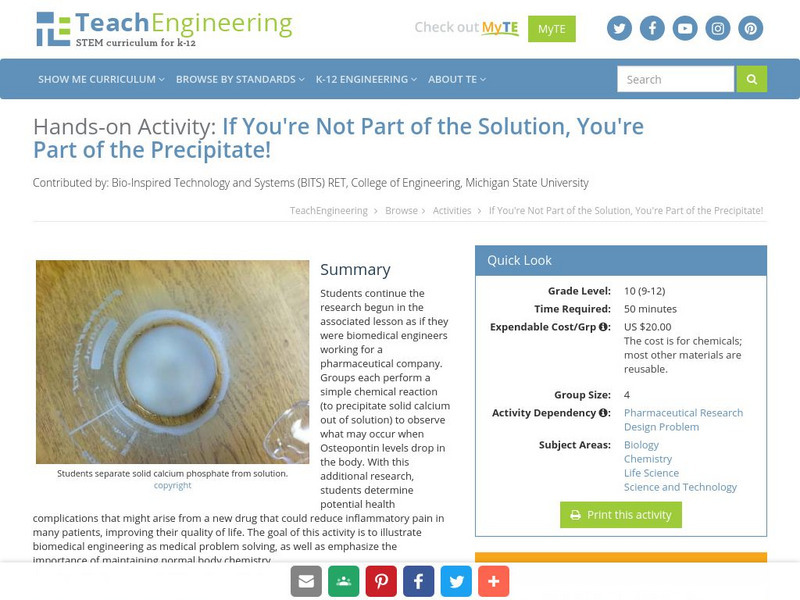
Teach Engineering: If You're Not Part of the Solution!
Students continue the research begun in the associated lesson as if they were biomedical engineers working for a pharmaceutical company. Groups each perform a simple chemical reaction (to precipitate solid calcium out of solution) to...
Khan Academy
Khan Academy: Introduction to Gravimetric Analysis: Volatilization Gravimetry
Introduction to volatilization gravimetry and precipitation gravimetry. An example using volatilization gravimetry to determine the purity of a metal hydrate mixture.
Georgia Department of Education
Ga Virtual Learning: Ap Chemistry: Acid Base and Solution Equilibrium
Through a variety of activities, students learn that not all acid and base reactions go to completion, and instead, they establish equilibrium. Acid-base equilibrium expressions have their own constants, ka and kb. Students will perform...
American Chemical Society
Middle School Chemistry: Chapter 6: Chemical Change
Twelve interactive chemistry lessons about chemical changes complete with handouts and animations.
Texas Instruments
Texas Instruments: Conductimetric Titration & Gravimetric Determination
Students use a Conductivity Probe to measure change in conductivity during a chemical reaction and determine the equivalence point of the reaction They determine the mass of the product and calculate the molar concentration of the...
Frostburg State University
General Chemistry Online: Ten Signs of Chemical Change
Resource provides the ten signs that tell when a chemical change has occured. Each sign has a detailed explanation.