American Chemical Society
Middle School Chemistry: Protons, Neutrons, and Electrons
Explore the particles that make up atoms: protons, electrons, and electrons.
American Chemical Society
Middle School Chemistry: Protons, Neutrons, and Electrons
Investigate why a charged object is attracted or repelled by another charged object. Explore the concept that the attraction between positive protons and negative electrons holds an atom together.
CK-12 Foundation
Ck 12: Nuclear Stability and Binding Energy
[Free Registration/Login may be required to access all resource tools.] In this lesson, students learn how scientists study the properties of stable nuclei in order to draw generalizations about what makes a nucleus stable. They look at...
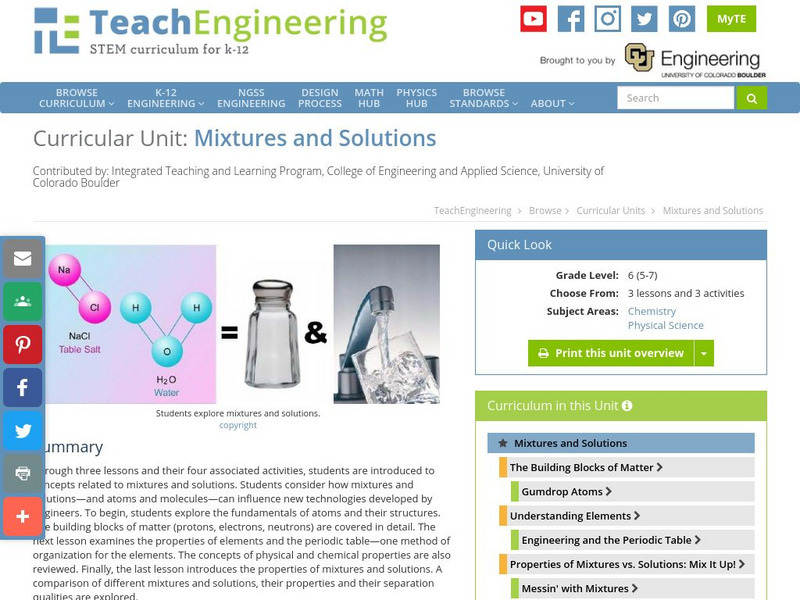
TeachEngineering
Teach Engineering: Mixtures and Solutions
This unit covers introductory concepts of mixtures and solutions. Students think about how mixtures and solutions, and atoms and molecules can influence new technologies developed by engineers. The first lesson explores the fundamentals...
Science Struck
Science Struck: How to Find Protons, Neutrons and Electrons
Brief explanations of how to determine how many protons, neutrons, and electrons are in an element.
California State University
Csudh Project for Chemistry: Protons, Electrons, and Neutrons
This page is an exercise in relating the number of protons, electrons, and neutrons for an atom or monoatomic ion.
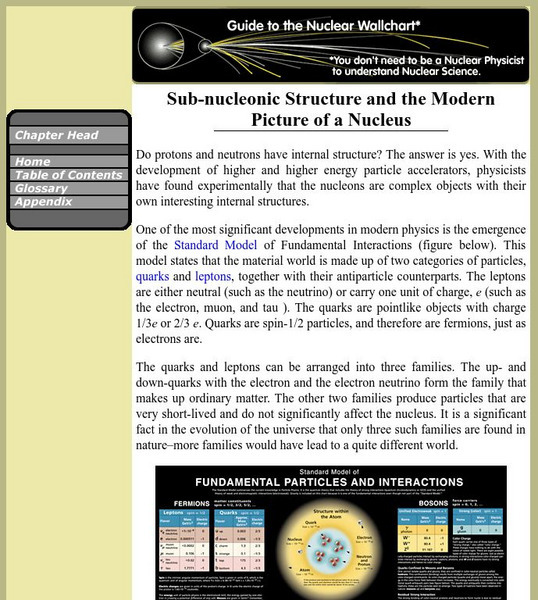
Lawrence Berkeley National Laboratory
Berkeley Lab: Sub Nucleonic Structure and the Modern Picture of a Nucleus
A simple explanation of the structures theoretically found within protons and neutrons. The resource consists of pictures and links to additional resources.
Scholastic
Scholastic: Study Jams! Science: Matter: Atoms: Protons, Neutrons, Electrons
A video and a short quiz on the parts of an atom, the periodic table, and molecules.
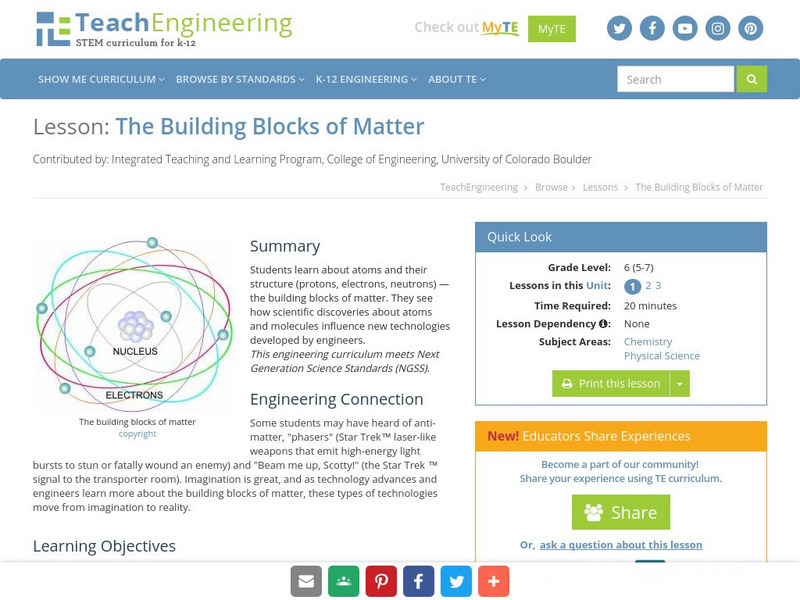
TeachEngineering
Teach Engineering: The Fundamental Building Blocks of Matter
This lesson plan explores the fundamentals of atoms and their structure. The building blocks of matter (protons, electrons, neutrons) are covered in detail. Students think about how atoms and molecules can influence new technologies...
Sophia Learning
Sophia: Subatomic Particles: The Neutron: Lesson 2
This lesson will explain that neutrons are particles in the nucleus that have no charge and a mass of one amu. It is 2 of 3 in the series titled "Subatomic Particles: The Neutron."
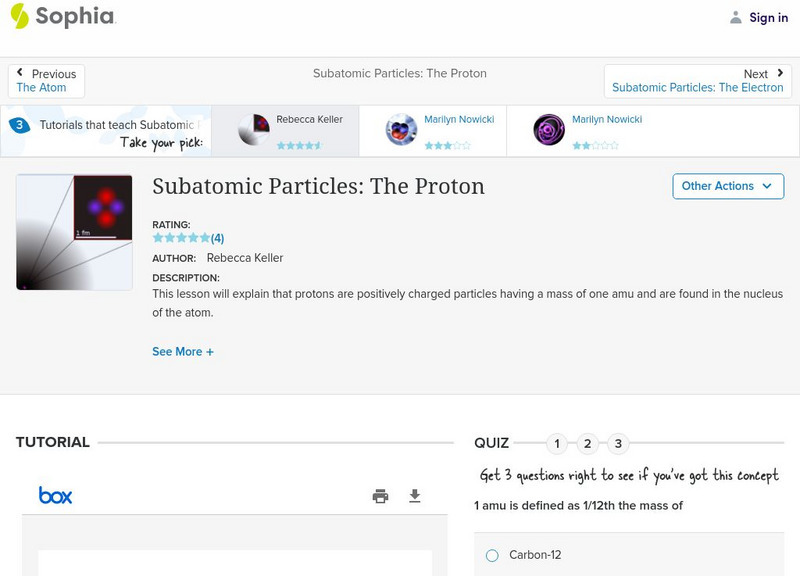
Sophia Learning
Sophia: Subatomic Particles: The Proton: Lesson 2
This lesson will explain that protons are positively charged particles having a mass of one amu and are found in the nucleus of the atom. It is 2 of 3 in the series titled "Subatomic Particles: The Proton."
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental: Element Math Game
The interactive activity examines the Periodic Table of Elements. Learners answer questions about the number of protons, electrons, neutrons, or nucleons that an atom of an element contains.
City University of New York
John Jay College of Criminal Justice: Atomic Structure
Resource shows the relationships bewteen terms such as atomic number, protons, and isotopes. Simple pictures of atoms help.
Science Education Resource Center at Carleton College
Serc: Proton Neutron Rotation and Nuclear Stability
In this activity, students will review and investigate the stability of the nucleus of various elements and determine factors that affect that stability. Students will get an understanding of why certain nuclei are unstable and what type...
Sophia Learning
Sophia: The Atom: Lesson 2
This lesson will illustrate that an atom is mostly empty space and has a positively charged, massive core (containing both protons and neutrons called the nucleus) surrounded by negatively charged electrons. It is 2 of 3 in the series...
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental Element Math Game!
Learn how to read the periodic table of elements as you solve these Math questions about the number of protons, neutrons, electrons or nucleons in an atom of an element. You can choose how many questions to answer, and how complex they...
Lawrence Berkeley National Laboratory
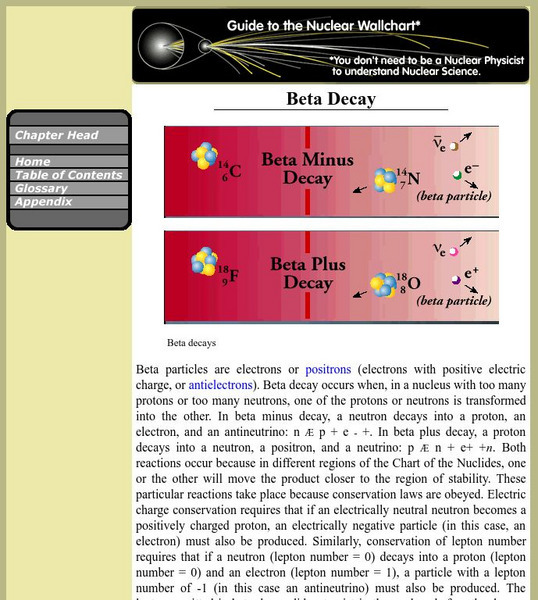
Berkeley Lab: Beta Decay
Entry explores the process of beta decay which occurs when, in a nucleus with too many protons or too many neutrons, one of the protons or neutrons is transformed into the other.
PBS
Nova: Atom Builder
Find out if you know enough about atoms to build them. The goal of the activity is to build an atom of a particular element by dragging the correct numbers of neutrons, protons and electrons into the atom.
Utah Education Network
Uen: Atom in a Bag
Learners will use bags of beads with known quantities of electrons, neutrons and protons to identify the element that they represent.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1923: Robert Andrews Millikan
This Nobel website on the life and scientific work of Robert A. Millikan includes a biography, images, and internet resources for further reading and research. Also included are the 1923 "Presentation Speech" which praised Millikan's...
BBC
Bbc: Gcse Bitesize: Atomic Structure
This lesson focuses on the structure of atoms. All substances are made from atoms. Each atom is made of a nucleus - containing protons and neutrons - surrounded by electrons. It provides a link to an assessment.
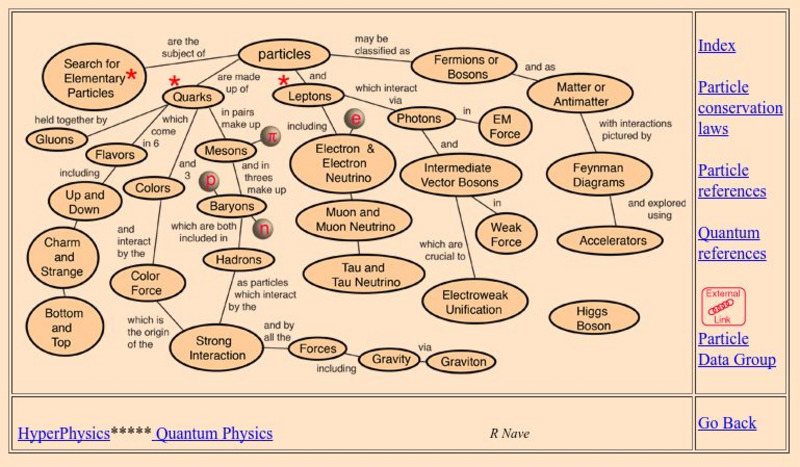
Georgia State University
Georgia State University: Hyper Physics: Particles
This is a very detailed site containing information on several sub-atomic particles including the Hadron.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1935 Presentation Speech
The Nobel Physics Chairman made this speech when presenting the Prize to Chadwick. It clearly explains the importance and depth of Chadwick's work. Site by Nobel e-Museum.
Other
University of Kansas: Quarked!: Matter Mechanic
Build elements and molecules using neutrons, protons, and electrons. Choices include helium, carbon, oxygen, aluminum, water, and salt.
Other popular searches
- Protons Neutrons Electrons
- Protons, Neutrons, Electrons
- Electrons, Neutrons, Protons
- Electrons, Protons, Neutrons
- Protons Neutrons, Electrons
- Atoms Neutrons Protons
- Protons Neutrons and Electrons
- Protons , Neutrons, Electrons