Thomas Jefferson National Accelerator Facility
Jefferson Lab: Beta Decay
This site from Jefferson Lab provides a description of beta decay along with two helpful formula examples. Several links are provided throughout this page for additional information on related subjects.
New York University
New York University: Law of Conservation of Energy
Site presents a straightforward presentation of Rutherford's work concerning the Law of Energy Conservation. Provides an adequate summary of early science on the nucleus, and includes much needed illustrations.
Sophia Learning
Sophia: Subatomic Particles: Lesson 3
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 3 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 4
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 4 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 6
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 6 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 7
This lesson will introduce the subatomic particles and explain where they are located and how they interact. It is 7 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: Lesson 5
Describe the difference between the subatomic particles, including their masses, locations, and charges. This lesson is 5 of 7 in the series titled "Subatomic Particles."
Sophia Learning
Sophia: Subatomic Particles: The Electron: Lesson 3
This lesson will explain that electrons are negatively charged particles with negligible mass and are found in pairs in orbitals surrounding the nucleus of an atom. It is 3 of 3 in the series titled "Subatomic Particles: The Electron."
Other
Fermi Laboratory:how Strong Is the Strong Force?
Use this site to learn about the four forces of nature. Also learn what determines the strength of a force. This question and answer site is a link of the Fermi National Accelerator Laboratory.
ClassFlow
Class Flow: Atomic Structure: Parts of the Atom
[Free Registration/Login Required] This flipchart defines the parts of the atom. It uses Activotes for assessment.
Science4Fun
Science4 Fun: What Is Atom
Fun and interesting illustrated information on atoms including composition, elements, and history.
Math Science Nucleus
Math/science Nucleus: Electrons and the Hairy Monster
This animation discusses electrons and the properties of electrons in a storybook format featuring hairy monsters, strange rocks, and fun animations.
Curated OER
[Bohr Model of Unununium]
A look at the transition metal unununium, which was renamed roentgenium in 2004. Includes basic information, including symbol, number, mass, melting and boiling points, and numbers of protons, electron, and neutrons.
Curated OER
Science Kids: Science Images: Basic Atom Structure
This diagram shows a basic atom structure with an atom core, proton, neutron and electron.
Lawrence Berkeley National Laboratory
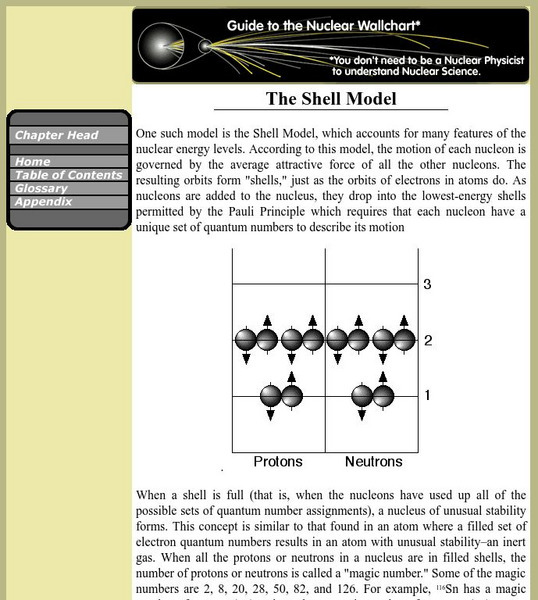
Berkeley Lab: The Shell Model
This entry investigates the Shell Model in which the motion of each nucleon is governed by the average attractive force of all the other nucleons.
Lawrence Berkeley National Laboratory
Berkeley Lab: Basic Nuclear Science Information
Site provides the ABC's of nuclear science including radioactivity and gamma decay to fission and comic rays.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: All About Atoms
The three basic particles that make up atoms are defined and illustrated.
Physics4kids
Physics 4 Kids: Where Traditional Physics Stops
We're about to move into the modern age of physics. In the early 1800's, scientists began examining the basis of matter, space, and time. Sometimes it gets very confusing, but the big idea is that Newton's physics describe about 90% of...
Sophia Learning
Sophia: Atomic Mass: Lesson 3
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 3 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Mass: Lesson 4
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. Module includes a slideshow and a quiz.
Sophia Learning
Sophia: Atomic Mass: Lesson 1
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 1 of 4 in the series titled "Atomic Mass."
Sophia Learning
Sophia: Atomic Number: Lesson 3
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 3 of 7 in the series titled "Atomic Number."
Sophia Learning
Sophia: Atomic Number: Lesson 6
This lesson explains what is represented by the atomic number, and how it varies from one element to the next. It is 6 of 7 in the series titled "Atomic Number."
Sophia Learning
Sophia: Atomic Mass: Lesson 2
This lesson explains what is represented by the atomic mass, and how it varies from one element to the next. It is 2 of 4 in the series titled "Atomic Mass."
Other popular searches
- Protons Neutrons Electrons
- Protons, Neutrons, Electrons
- Electrons, Neutrons, Protons
- Electrons, Protons, Neutrons
- Protons Neutrons, Electrons
- Atoms Neutrons Protons
- Protons Neutrons and Electrons
- Protons , Neutrons, Electrons