Science Struck
Science Struck: What Makes Up an Atom?
Describes the structure of an atom and the characteristics of the electrons, neutrons, and protons inside it. Includes some interesting facts about atoms.
Concord Consortium
Concord Consortium: Atom and Ion Builder
Explore how changing the numbers of protons, neutrons, and electrons affect the type of atom.
Purdue University
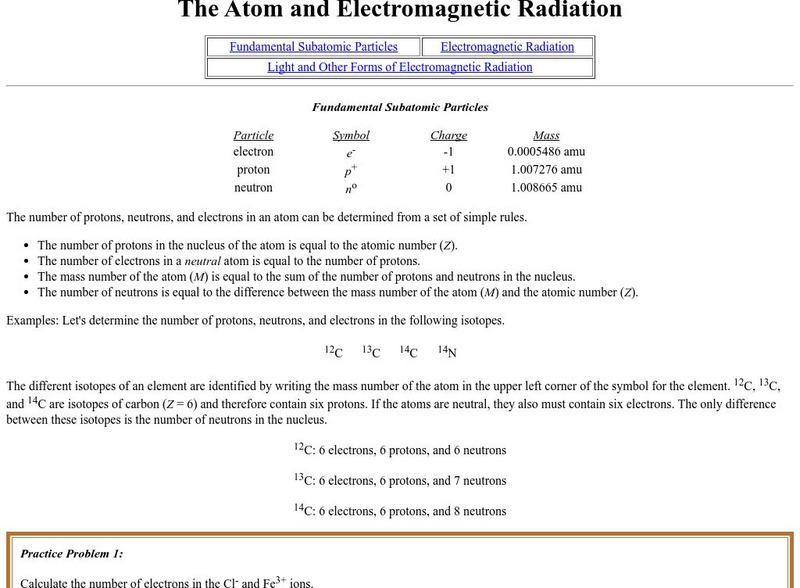
Purdue University: Fundamental Subatomic Particles
At this site from the Purdue University, the elementary subatomic particles are described and electromagnetic radiation is detailed. Includes learning exercises and answers.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: It's Elemental Element Math Game!
Learn how to read the periodic table of elements as you solve these Math questions about the number of protons, neutrons, electrons or nucleons in an atom of an element. You can choose how many questions to answer, and how complex they...
Concord Consortium
Concord Consortium: Stem Resources: Atomic Structure
Introduces learners to atomic models of the past and present, focusing on the orbital model and an explanation of its basis. Learners then have the opportunity to "make an atom" and contrast it with an ion, followed by an isotope. The...
Ducksters
Ducksters: Science for Kids: The Atom
Kids learn more about the science of the atom. Electrons, neutrons, and protons make up the smallest bits of matter.
CK-12 Foundation
Ck 12: Structure of the Atom
[Free Registration/Login may be required to access all resource tools.] Students learn about the important discoveries of subatomic particles, and how they led to our current understanding of the atom.
ClassFlow
Class Flow: Element Math
[Free Registration/Login Required] In this flipchart students determine the number of protons, neutrons, and electrons of elements essential to life. They use Activotes to check knowledge gained. Students create a Bohr Model.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Physics 1935 Presentation Speech
The Nobel Physics Chairman made this speech when presenting the Prize to Chadwick. It clearly explains the importance and depth of Chadwick's work. Site by Nobel e-Museum.
Other
University of Kansas: Quarked!: Matter Mechanic
Build elements and molecules using neutrons, protons, and electrons. Choices include helium, carbon, oxygen, aluminum, water, and salt.
Tom Richey
Slide Share: Atomic Structure
Slideshow looking at the history of models of the atom, including those proposed by John Dalton, J.J. Thomson, Ernest Rutherford, Niels Bohr, and James Chadwick. Discusses subatomic particles, including the numbers of protons, neutrons,...
Estrella Mountain Community College
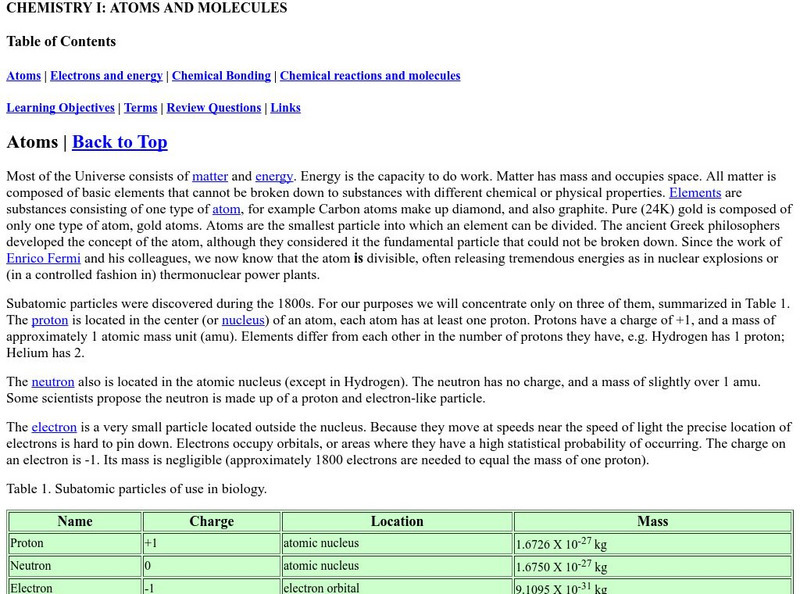
Online Biology Book: Chemistry I: Atoms and Molecules
In this online biology textbook, learn about atoms and molecules as they relate to life. Find out about topics such as electrons and energy, chemical bonding, and chemical reactions.
American Chemical Society
Middle School Chemistry: Chapter 4: The Periodic Table and Bonding
Six middle school chemistry lessons about the periodic table and bonding complete with handouts and animations.
PBS
Pbs Learning Media: The Atom
In this interactive activity from ChemThink, students will take a closer look at atomic structure, properties, and behaviors. Includes background reading material and discussion questions.
PBS
Pbs Learning Media: Atomic Structure
Take a look at the parts of an atom and learn about its properties.
Science Education Resource Center at Carleton College
Serc: Tic Tac Toe Pick 3 in a Row Atomic Model Assignment
A choice menu assignment where young scholars select between several extension activities that help understand the structure of the atom.
Science Education Resource Center at Carleton College
Serc: Drawing Atoms
This activity serves as an introduction to chemistry, and can be used to help young scholars draw a two dimensional image of the atom.
Sophia Learning
Sophia: Subatomic Particles: Lesson 2
Describe the $ifference between the subatomic particles, including their masses, locations, and charges. This lesson is 2 of 7 in the series titled "Subatomic Particles."
CK-12 Foundation
Ck 12: Chemistry: Atomic Nucleus
[Free Registration/Login may be required to access all resource tools.] Describes the modern concept of the atomic nucleus.
Soft Schools
Soft Schools: Subatomic Particles Quiz
Take this interactive, multiple-choice quiz over subatomic particles, then review your score and any missed questions at the end.
Other
Particle Adventure Dutch Version
Dutch version of the well-known "Particle Adventure" physics website that teaches students about atoms, mass, particle physics, and quantum physics. The site discusses theories related to physics and provides other links related to the...
Lawrence Berkeley National Laboratory
Berkeley Lab: La Aventura De Las Particulas
Learn the fundamentals of particles and forces with this site. Explore the paths that explain matter in the universe.
Mocomi & Anibrain Digital Technologies
Mocomi: Structure of an Atom
An atom is made of three parts - protons, neutrons, and electrons. Here you can explore these different parts.
Thomas Jefferson National Accelerator Facility
Jefferson Lab: Beta Decay
This site from Jefferson Lab provides a description of beta decay along with two helpful formula examples. Several links are provided throughout this page for additional information on related subjects.
Other popular searches
- Protons Neutrons Electrons
- Protons, Neutrons, Electrons
- Electrons, Neutrons, Protons
- Electrons, Protons, Neutrons
- Protons Neutrons, Electrons
- Protons Neutrons and Electrons
- Protons , Neutrons, Electrons