Chemistry Collective
Chem Collective: Determining Stoichiometric Coefficients
Students use the virtual lab to determine how four unknown substances react with each other including their stoichiometric coefficients. In this randomized activity, each student receives a different reaction and students can check their...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Chemical Calculations and Chemical Equations [Pdf]
Slide show focusing on the elements of stoichiometry relating to limiting reactants. Go through the first several slides to review or introduce the topic.
Chemistry Collective
Chem Collective: Textbook Style Limiting Reagents Problems
Textbook-style practice limiting reagent exercises with that can be used as a way to "predict and check" your answers using the virtual lab.
Chemistry Collective
Chem Collective: Textbook Style Limiting Reagents Problem Ii
In this activity, students practice with experiments involving limiting reagents and the test their knowledge to determine the concentration of an unknown solution.
Science Education Resource Center at Carleton College
Serc: How Big Is the Balloon?
A chemistry lab where students investigate limiting reagents and balanced chemical equations. A simple experiment that gives students a physical example of what limiting reactant means.
Crescent Public Schools
The Internet Science Room: Stoichiometry
This chemistry tutorial presents Stoichiometry to students with explicit steps for solving a mass-mass problem and for solving a limiting reactant problem.
Concord Consortium
The Concord Consortium: Molecular Workbench: Seeing Chemical Equilibrium
Observe a visual representation of the equilibrium of products and reactants in chemical reactions. Record data while the reactions are taking place and print out a report afterwards.
Sophia Learning
Sophia: Changes in Concentration at Equilibrium
This lesson will provide examples of how to identify an equilibrium shift due to a change in concentration of either reactants or products.
Sophia Learning
Sophia: Chemical Reactions: Stoichiometry
This lesson on stoichiometry demonstrates how to use a balanced chemical equation to determine the mass of a product given the starting mass of a reactant. [7:55]
Other
Nearpod: Photosynthesis
In this lesson on exploring photosynthesis, learners will explore photosynthesis by examining the reactants and products of this process.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Real World Applications of Equation Stoichiometry
This audio book, narrated by author Mark Bishop, describes how equation stoichiometry can be used in the real world. Many examples are given to help understand how to plan chemical reactions, solve for limiting reactants, and calculate...
BBC
Bbc: Gcse Bitesize: Chemical Calculations
You should be able to calculate the masses of reactants and products from balanced equations, and the formula of a compound from information about reacting masses.
CK-12 Foundation
Ck 12: Plix: Balancing Chemical Reactions
[Free Registration/Login Required] Demonstrate a normal chemical reaction by dragging atoms from the products to create the reactants in this chemical equation.
Khan Academy
Khan Academy: Limiting Reagents and Percent Yield
How to determine the limiting reagent, and using stoichiometry to calculate the theoretical and percent yield.
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Equilibrium Pressure of Reactants and Products
Learn about the equilibrium pressures of chemical reactions. Follow the general steps used in calculating the pressures, and see some examples. Also find links to tutorials, presentation, and animations about other chemistry topics.
Frostburg State University
Frostburg State University: Limiting Reagents
Middle of a slide show on stoichiometry that shows a nice graphical look and example of limiting reagents.
Shodor Education Foundation
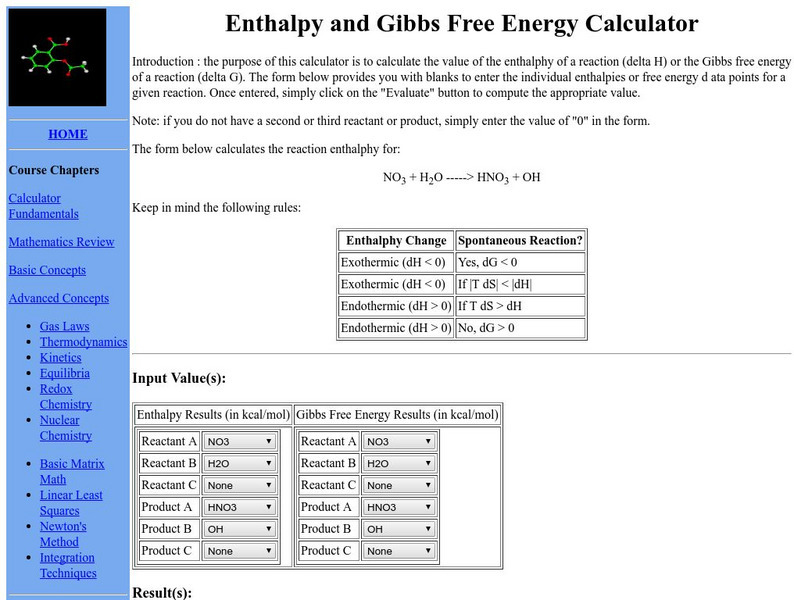
Shodor: Enthalpy and Gibbs Free Energy Calculator
Interactive calculator that allows you to input reactants and products of a reaction and calculate the change in enthalpy and Gibbs Free Energy for the reaction.
Dartmouth College
Dartmouth College: Chem Lab: Titration
A titration is a method of analysis that will allow you to determine the precise endpoint of a reaction and therefore the precise quantity of reactant in the titration flask. This site demonstrates how to correctly perform this analysis.
CK-12 Foundation
Ck 12: Types of Chemical Reactions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will define and give general equations for combination, decomposition, single-replacement, and double-replacement...
CK-12 Foundation
Ck 12: Chemical Equations
[Free Registration/Login may be required to access all resource tools.] Students explore mass relations between reactants and products for a given chemical process, and practice balancing chemical equations.
CK-12 Foundation
Ck 12: Types of Chemical Reactions
[Free Registration/Login may be required to access all resource tools.] In this learning module, studnets will classify a chemical reaction as a combination, decomposition, single replacement, double replacement, or combustion reaction....
Khan Academy
Khan Academy: The Equilibrium Constant K
Reversible reactions, equilibrium, and the equilibrium constant K. Learn how to calculate K, and how to use K to determine if a reaction strongly favors products or reactants at equilibrium.
TeachEngineering
Teach Engineering: Reaction Exposed: The Big Chill!
Students investigate the endothermic reaction involving citric acid, sodium bicarbonate and water to produce carbon dioxide, water and sodium citrate. In the presence of water [H2O]; citric acid [C6H8O7] and sodium bicarbonate [NaHCO3]...
Chemistry Collective
Chem Collective: Camping Problem Ii
In this part of the MRE scenario, students determine change in the enthalpy of a reaction as the concentration of reactants are varied.
Other popular searches
- Reactants and Products
- Limiting Reactants
- Chemistry Limiting Reactants
- Reactants of Photosynthesis
- Reactants With Vinegar
- Mass of Reactants
- Excess Reactants
- Introduction to Reactants
- Reactants of Equations


![Chiral Publishing: An Introduction to Chemistry: Chemical Calculations and Chemical Equations [Pdf] PPT Chiral Publishing: An Introduction to Chemistry: Chemical Calculations and Chemical Equations [Pdf] PPT](https://d15y2dacu3jp90.cloudfront.net/images/attachment_defaults/resource/large/FPO-knovation.png)