Encyclopedia Britannica
Encyclopedia Britannica: Erwin Schrodinger
This site gives brief biographical information about Austrian physicist, Erwin Schrodinger.
Encyclopedia Britannica
Encyclopedia Britannica: Linus Pauling
This is a brief biography of Linus Pauling, the famous American chemist and two time Nobel Prize winner.
Michael Blaber, PhD
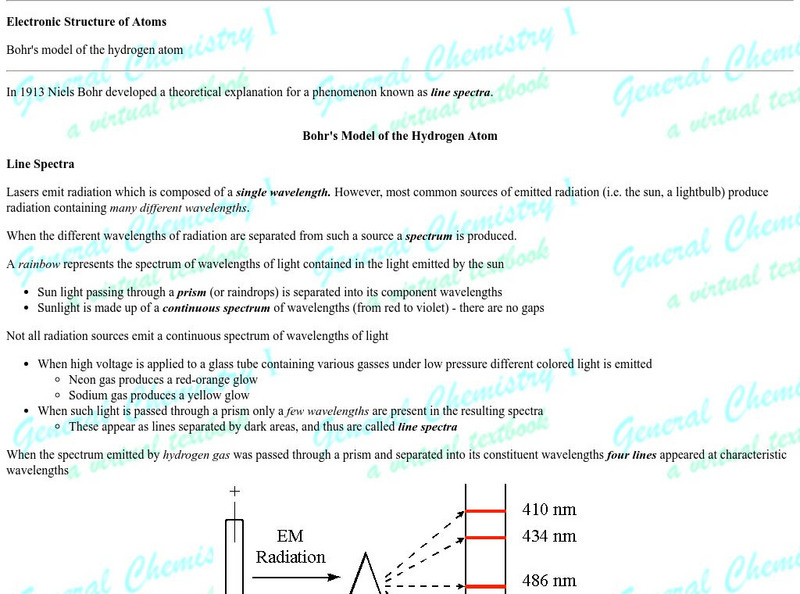
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Concord Consortium
Concord Consortium: Stem Resources: Excited States and Photons
Students are able to explore the effects of energy on excitation of atoms through simulations, then relate this information to photons at atoms' absorption and emission of light. Multiple-choice and short answer questions are found...
Federation of American Scientists
Fas: National Security Policy Arms Control Disarmament
A lengthy essay describing the negotiations and politics involved in President Kennedy's quest to create a permanent ban on nuclear tests.
Simon Fraser University
Chem1 Virtual Textbook: What Is a Wave?
Acting as part of an overview on quantum theory, this section of the site answers the question, what is a wave? In addition to a definition, several examples and formulas are provided.
Simon Fraser University
Chem1 Virtual Textbook: Electrons
Acting as part of an overview on quantum theory, this section of the site deals with questions related to electrons, such as "If the electron cannot be localized, can it be moving?" and "Why does the electron not fall into the nucleus?"
Friesian School
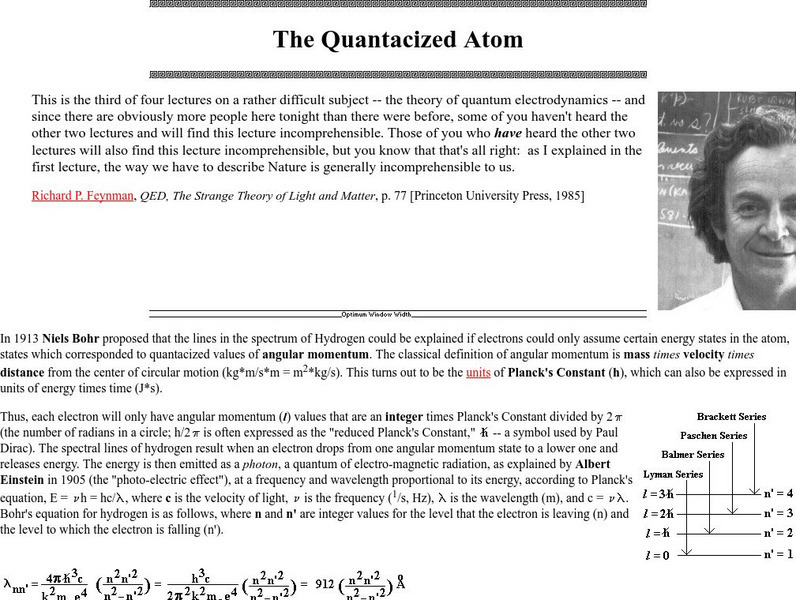
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Lawrence Berkeley National Laboratory
Berkeley Lab: Particle Adventure: The Standard Model
An introduction to the Standard Model, a theory which attempts to explain atomic structure using leptons, quarks, and force carrier particles.
University of St. Andrews (UK)
University of St. Andrews: Insulators
The nature of insulators is described at the atomic level. Band gap theory is used to explain what distinguishes insulators from conductors.
University of California
Ucla: Maria Goeppert Mayer
UCLA Physics presents three documents here on Maria Goeppert Mayer, including a brief biography, a longer biography, and a physics meeting address on Mayer and the specifics of her work in nuclear physics.
Wikimedia
Wikipedia: Mutual Assured Destruction
A complete description of the concept of Mutual Assured Destruction. Explains what the concept means, and how the concept was used during the Cold War and up to the George W. Bush Administration. Includes links to terms that may require...
Towson University
Towson University: Shapes of Molecules
This chemistry class printout details the main points of molecular geometry and explains bond hybridization and bond angles.
University of Maryland
University of Maryland: Optics, Electromagnetic Waves
This site from the University of Maryland provides part of an anecdotal history of optics and the study of light. Extremely thorough treatment of how scientists came to believe in the wave nature of light, the idea of an electromagnetic...
Other
Indiana University Northwest: The Aufbau Principle
The Aufbau Principle defined. Site contains charts, graphs, and examples to help in your learning of the Principle and its many purposes.
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Proceedings of the Friesian School/the Quantacized Atom
A very lengthy page from friesian.com discussing Bohr's theory of electronic energy levels and the explanation of commonly observed atomic emission line spectra. The concept of a photon and Einstein's observation of the photoelectric...
Curated OER
Bbc Mundo: El Ano Magico De Einstein
A special feature on Einstein from the BBC, in Spanish. Follow Einstein's life, including his role in the development of the atomic bomb; learn about the impact and practical applications of his work; or watch a speech he gave in 1950...