Science Education Resource Center at Carleton College
Serc: Why Is the Earth Still Hot Inside?
For this lesson, learners conduct heat transfer experiments to investigate why the Earth is still hot at its core, even after billions of years since its formation. They will learn that the rate of heat transfer is dependent on an...
TOPS Learning Systems
Tops Learning Systems: Top Science: Conduction and Convection [Pdf]
Experiment investigating conduction and convection of heat in water.
Exploratorium
Exploratorium: Science Snacks: Pie Pan Convection
In this experiment, students observe what happens when a pan of soapy, colored water is heated. They will see that convection currents cause the fluid to rise and sink in a localized convection cell.
Exploratorium
Exploratorium: Give and Take
A description of a museum exhibit that compares the heat radiated from two pieces of metal--one painted black and the other shiny. Great idea stimulus for a student project or lab investigation.
MadSci Network
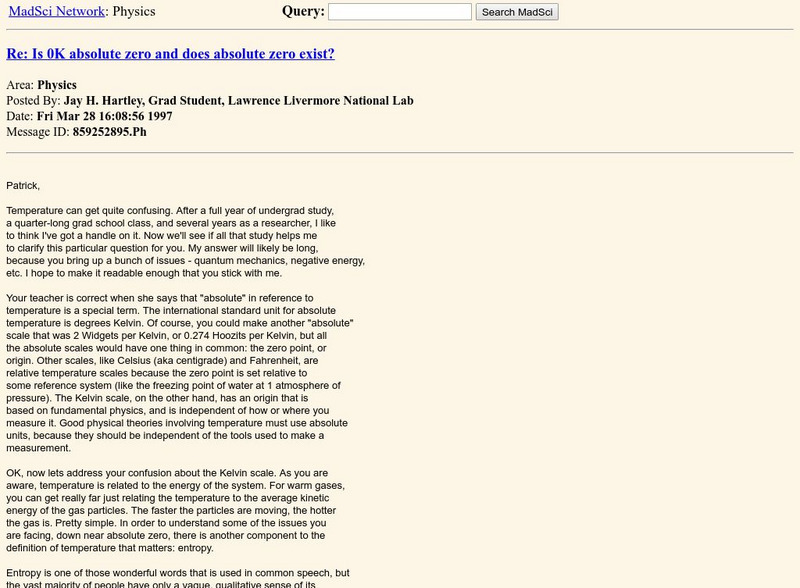
The Mad Scientist Network/is 0 K Absolute Zero?
Using a question and answer format, this page explains what is meant by the term "absolute zero." The basis for the Kelvin temperature scale is described. And the relationship between heat or energy and temperature is discussed.
BBC
Bbc Schools: Ks2 Bitesize: Science: Materials: Keeping Warm
Easy-to-understand overviews, with illustrations, a game, and quiz questions, about thermal conductors and insulators.
Khan Academy
Khan Academy: Thermodynamics Article
Thermodynamics is a very important branch of both physics and chemistry. It deals with the study of energy, the conversion of energy between different forms and the ability of energy to do work. Through this article, you will begin to...
FT Exploring
Ft Exploring: The Second Law of Thermodynamics
Learn about one of the most misunderstood principles of physics, the second law of thermodynamics.
University of Sydney (Australia)
Thermal Physics Module: Ideal Gases [Pdf]
A molecular model of a gas is introduced and explained. Assumptions behind the ideal gas law are presented. The ideal gas law is stated. Charles' law and Boyle's law are derived from the ideal gas law.
Georgia State University
Georgia State University: Hyper Physics: Zeroth Law of Thermodynamics
The principle of thermal equilibrium is discussed and explained. The zeroth law of thermodynamics is stated. Links to several other pages with related information are provided.
Georgia State University
Georgia State University: Hyper Physics: Radiation Cooling of Body
Discusses the means by which the body regulates its temperature. The role of radiation in this process is explained. An equation for calculating the rate at which energy is transferred by radiation is presented. Also, an interactive...
Sophia Learning
Sophia: Identify Enthalpy of Reaction
A video tutorial explaining the enthalpy of a reaction using an energy diagram. [2:42]
TeachEngineering
Teach Engineering: Hot Cans and Cold Cans
Students apply the concepts of conduction, convection, and radiation as they work in teams to solve two problems. One problem requires that they maintain the warm temperature of one soda can filled with water at approximately body...
Georgia State University
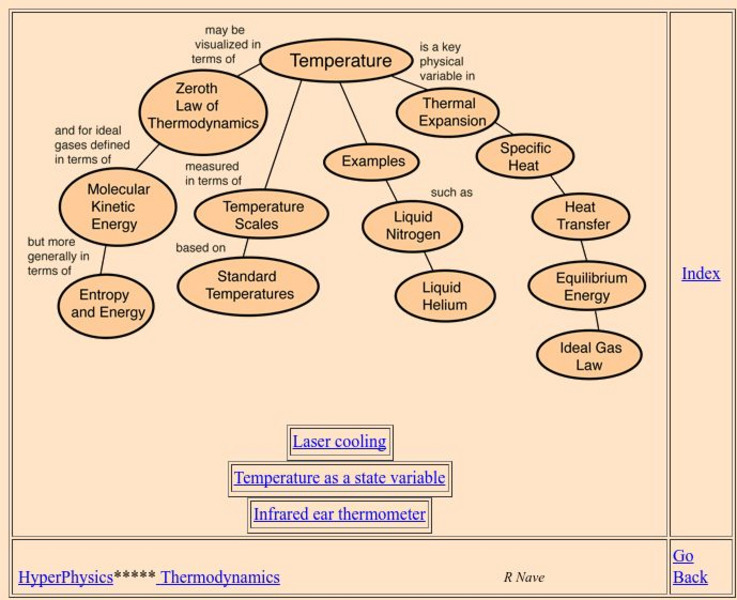
Georgia State University: Hyper Physics: Temperature Concepts
An indexing page which includes links to a wealth of pages detailing the conceptual meaning of temperature. A hypertext format allows the visitor to quickly gain access the desired information.
Georgia State University
Georgia State University: Hyper Physics: Area Expansion
The concept of area expansion is presented and explained. An equation for calculating the amount of area expansion is provided.
Georgia State University
Georgia State University: Hyper Physics: Temperature
A page describing the concept of temperature and temperature scales. An interactive JavaScript form allows the visitor to investigate the relationship between the Kelvin, Celsius and Fahrenheit scales; enter a value and allow the...
Georgia State University
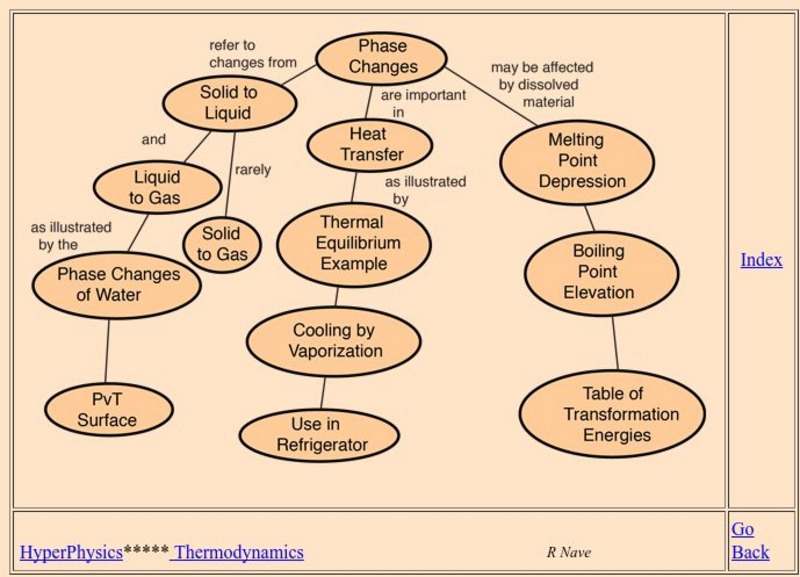
Georgia State University: Hyper Physics: Phase Change Concepts
An indexing page for the HyperPhysics site. This page includes links to a variety of pages at the site which contain information related to phase changes. Each individual page consists of informative graphics and clear explanations.
Other
Rob's North York Moors Railway: The Steam Locomotive
A page describing the parts of a steam engine and explaining their operation. Thorough discussion of the parts.
Colorado State University
Csu: Model of Basic Otto Cycle
This site from the Colorado State University discusses the Otto cycle of a car engine. Includes a highly mathematical treatment of the efficiency of such engines. Includes a link to a java applet investigating the efficiency of such...
Georgia State University
Georgia State University: Hyper Physics: Law Concepts
This site from Georgia State University Department of Physics and Astronomy is an indexing page for the HyperPhysics site. The page links to a variety of other pages which discuss concepts related to entropy and the second law of...
Georgia State University
Georgia State University: Hyper Physics: Entropy
This informative site is from Georgia State University. The tendency of nature to move towards a more disordered state in an isolated system, a concept known as entropy, is discussed. This concept is well-depicted in meaningful diagrams.
University of Colorado
University of Colorado: Physics 2000: Temperature and Absolute Zero
Another awesome page from the Physics 2000 site. Entertaining, interactive, educational, understandable. Explains the meaning of temperature and absolute zero. Discusses the temperature scales. Requires Java.
Science Buddies
Science Buddies: Bake Your Ice Cream
This activity will teach you how it is even possible to bake ice cream in a hot oven and have it come out frozen.
Science Education Resource Center at Carleton College
Serc: Exploring Molecular Movement: Does Temperature Matter?
In this interactive demonstration, students observe what happens to food coloring when dropped into beakers containing different temperatures of water.



![Tops Learning Systems: Top Science: Conduction and Convection [Pdf] Activity Tops Learning Systems: Top Science: Conduction and Convection [Pdf] Activity](https://d15y2dacu3jp90.cloudfront.net/images/attachment_defaults/resource/large/FPO-knovation.png)