University of Sydney (Australia)
Entropy and the Second Law of Thermodynamics [Pdf]
A set of printable pdf pages from the University of Sydney's "Thermal Physics Module" site. Entropy is defined and explained conceptually. The mathematical treatment of entropy is introduced. The second law of thermodynamics and its...
CK-12 Foundation
Ck 12: Heat Flow
[Free Registration/Login may be required to access all resource tools.] In this lesson, students study the difference between reactions that absorb versus release heat as well as how to measure this change in energy.
University of Sydney (Australia)
Thermal Physics Module: Ideal Gases [Pdf]
A molecular model of a gas is introduced and explained. Assumptions behind the ideal gas law are presented. The ideal gas law is stated. Charles' law and Boyle's law are derived from the ideal gas law.
Wikimedia
Wikipedia: The Black Hole
This site examines the black hole as an object in astrophysics. Delve into this comprehensive resource that covers this concept from its history, to qualitative physics, the reality of black holes, mathematical physics and more.
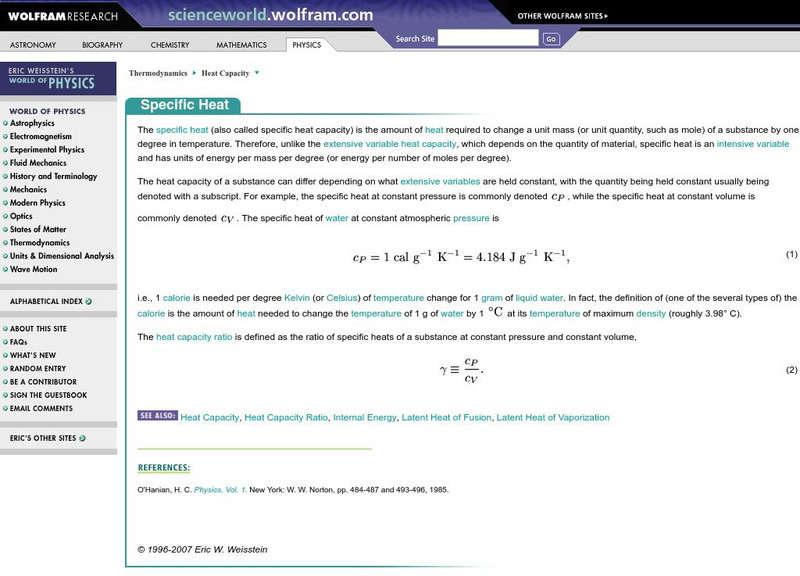
Wolfram Research
Wolfram Science World: Specifiic Heat
This site has information on specific heat,the amount of heat required to change a unit mass of a substance by one degree in temperature. Included are many links and formulas.
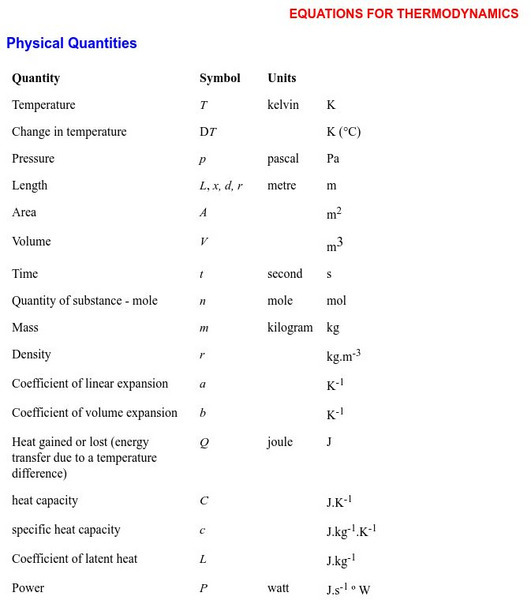
University of Sydney (Australia)
Equations for Thermodynamics
An exhaustive list of equations and formulas which are commonly used in thermal physics (including equations for triple point). Equations are organized according to category. Meaning of the symbols is clearly stated.
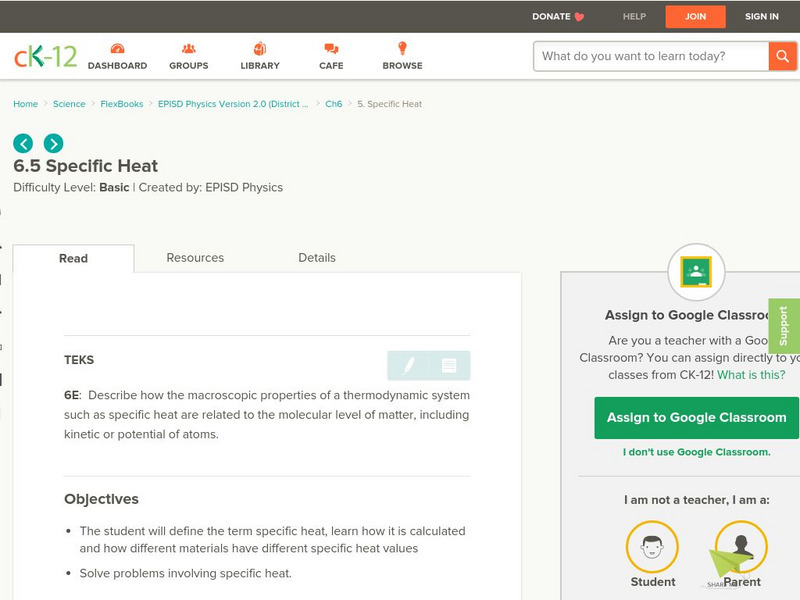
CK-12 Foundation
Ck 12: Specific Heat
[Free Registration/Login may be required to access all resource tools.] Students explore the concept of specific heat, learn how it is calculated, and find out how different materials have different specific heat values.


![Entropy and the Second Law of Thermodynamics [Pdf] Activity Entropy and the Second Law of Thermodynamics [Pdf] Activity](https://d15y2dacu3jp90.cloudfront.net/images/attachment_defaults/resource/large/FPO-knovation.png)