Frostburg State University
General Chemistry Online: The Mole Concept
Resource provides notes on Moles and Stoichiometry. Deals with all book-keeping aspects, including as section on yields and limiting reactants. Includes lesson plans, lecture slides and notes, links to related websites, and frequently...
Frostburg State University
General Chemistry Online: Faq: Acids and Bases
Click the basic questions regarding acids, bases, and pH to view the answers. There are sections on basic concepts, buffer solutions, pH calculation, equilibrium constants, indicators, neutralization reactions, and titrations.
Frostburg State University
General Chemistry Online: Atoms & Ions
This site from the General Chemistry Online of the Frostburg State University provides a review of the history of atomic theory, the discovery of the electron, and the discovery of the nucleus. Details on weighing atoms, ion charges,...
Frostburg State University
Why Does the Solubility of Gases Usually Increase as Temperature Goes Down?
This chemistry course article, "Why does the solubility of gases usually increase as temperature goes down?," provides text and related links on the effects of temperature and pressure on the solubility of gases.
Frostburg State University
Frostburg State University Chemistry Online: Viscosity and Paste
Describes the changes in viscosity when various amounts of force are applied to a Non-Newtonian liquid. Gives the example of cornstarch paste.
Frostburg State University
Frostburg State University: General Chemistry Online
Resource provides information about calculating molality.
Frostburg State University
General Chemistry: Gaseous Equation of State Calculator
A JavaScript calculator which allows one to investigate the distinction between an ideal gas and a real (Van der Waal or Dieterici) gas. State variables can be entered and an update on the values of other variables are given (as...
Frostburg State University
General Chemistry Online: Molecules and Compounds
A complete lesson on compounds, from introductory material in the beginning to a practice exam at the end. This lesson covers bonding, ionic compounds, molecular compounds, naming compounds, structural formulas, polyatomic ions, and much...
Frostburg State University
Frostburg State University Chemistry Online: Polyatomic Ions
A good reference page for anyone studying polyatomic ions. Tables included on this page organize polyatomic ions by family and by charge. Information on naming compounds with polyatomic ions is also provided. There is a link to an online...
Frostburg State University
General Chemistry Online: The Poisoned Needle
A strange bird phenomenon first occurred in a small California town in 1961. Uncover how separation technologies were used to solve the amnesic shellfish poisoning mystery.
Frostburg State University
General Chemistry Online: Physical and Chemical Changes
Resource provides information about the difference between physical and chemical changes. Includes a definition and examples of each.
Frostburg State University
General Chemistry Online: Faq the Mole Concept
This FAQ site over general chemistry concepts presents the question: "Why are moles used?" It explains what moles are and how they can be used to count molecules and how grams can be turned into moles.
Frostburg State University
General Chemistry Online: The Periodic Table
This is a teacher's companion guide with lesson plans for the periodic table. It includes learning objectives, lecture notes, links to related sites, answers to frequently asked questions, and a glossary of related terms.
Frostburg State University
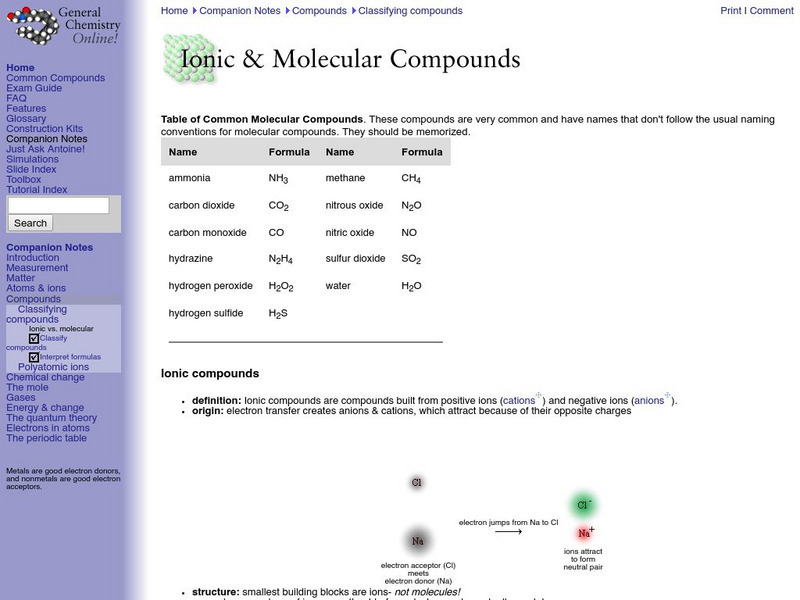
General Chemistry Online: Ionic and Molecular Compounds
Provides a good outline of the concepts involved in ionic and covalent bonding, with links to definition of terms. Features a list of common molecular compounds and a chart that compares ionic and molecular compounds.
Frostburg State University
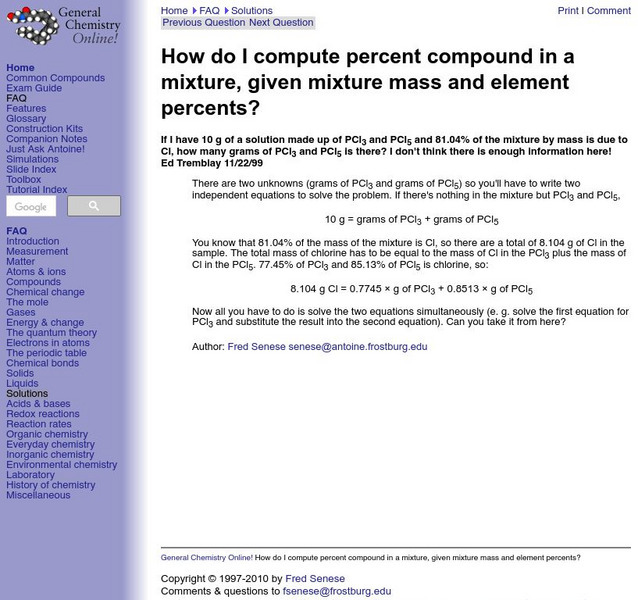
General Chemistry: Percent Compound in a Mixture
Resource contains and example problem and solution of how to compute percent compound within a mixture when given the mixture's mass and element percents.
Frostburg State University
General Chemistry Online: Ten Signs of Chemical Change
Resource provides the ten signs that tell when a chemical change has occured. Each sign has a detailed explanation.
Frostburg State University
General Chemistry Online: Glossary: Polymer Chemistry
A glossary of terminology surrounding polymer chemistry. Good site for vocabulary building or reinforcement.
Frostburg State University
University of Frostburg: How Nonpolar Molecules Dissolve
This site from the University of Frostburg provides an explanation of the process by which nonpolar molecules dissolve in water.
Frostburg State University
General Chemistry Online: Water to Wine
The molecular basis of indicator color changes. Includes Chime structures for the acid and base forms of phenolphthalein, methyl orange, red cabbage indicator, and cyanidin diglucoside.
Frostburg State University
General Chemistry Online: Anandamide
Is there actually a chemical that makes us feel bliss when we consume something like chocolate? The messenger molecule, anandamide, allows scientists and the general public to connect the worlds of human behavior and molecular science.
Frostburg State University
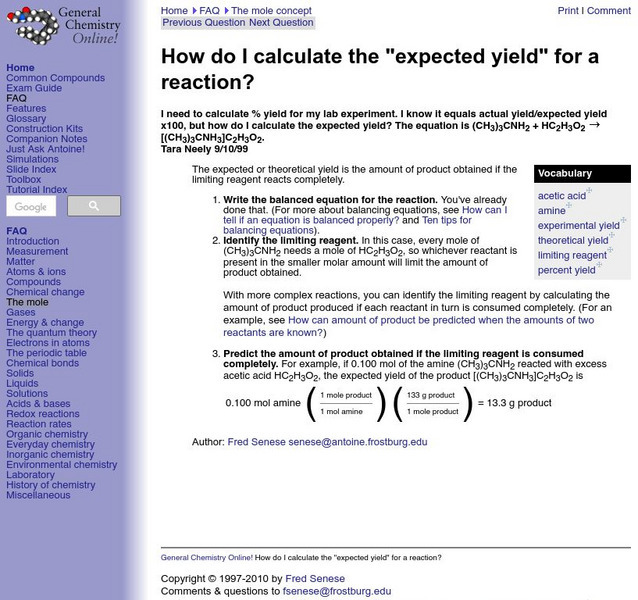
General Chemistry Online: Expected Yield
Frostburg State University provides an example of a problem dealing with how to find expected yield. Includes step-by-step directions on how to solve the problem.
Frostburg State University
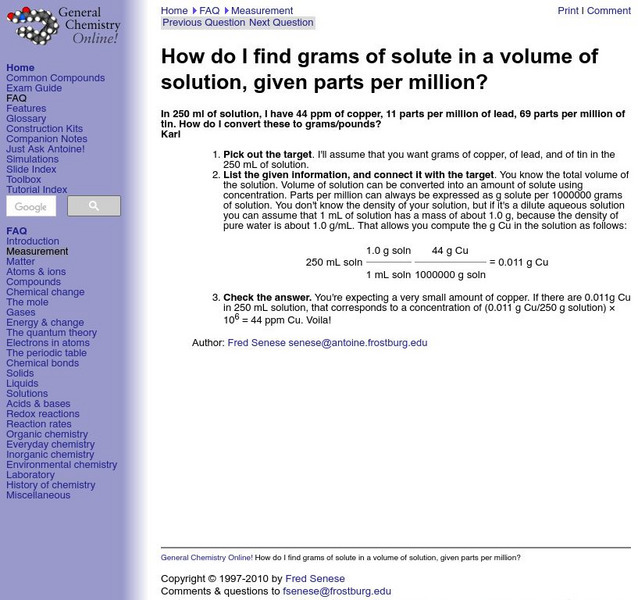
Frostburg State University: Grams of Solution
Resource presents the answer to "How do I find grams of solute in a volume of solution, given parts per million?" in an easy-to-understand format.
Frostburg State University
General Chemistry Online: How Can Molarity Be Converted
Resource provides information about converting molarity to normality.
Frostburg State University
Frostburg State Chemistry Online: Water Bond Angles & Phase Changes
Describes the bond angle of the water molecule during the transition from liquid to gas state. Also provides several examples and explanations for responses and answers given.