Curated by
ACT
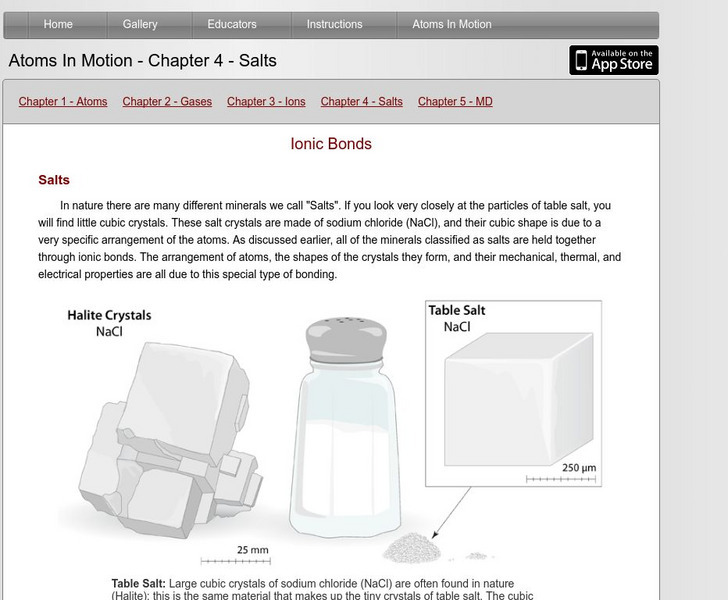
In nature there are many different minerals we call "Salts". If you look very closely at the particles of table salt, you will find little cubic crystals. These salt crystals are made of sodium chloride (NaCl), and their cubic shape is due to a very specific arrangement of the atoms. As discussed earlier, all of the minerals classified as salts are held together through ionic bonds. The arrangement of atoms, the shapes of the crystals they form, and their mechanical, thermal, and electrical properties are all due to this special type of bonding.
3 Views
0 Downloads
Concepts
Additional Tags
Classroom Considerations
- Knovation Readability Score: 4 (1 low difficulty, 5 high difficulty)
- This resource is only available on an unencrypted HTTP website.It should be fine for general use, but don’t use it to share any personally identifiable information