Curated and Reviewed by
Lesson Planet
This Concentrating Charge and Electric Fields interactive also includes:
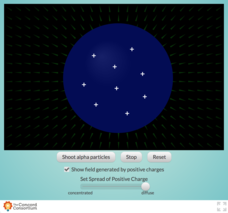
How did Rutherford determine that the nucleus was the center of an atom? Take a look inside the famous Gold Foil Experiment with an interesting interactive. Learners fire a beam of alpha particles at a nucleus containing variable concentrations of positively charged protons. They then observe the effects of the electric field upon the particles.
7 Views
2 Downloads
Concepts
Additional Tags
Instructional Ideas
- Use the interactive as the basis for model development to study the behavior of objects moving through a magnetic field
- Have the class record their observations for concentrated, medium, and diffuse charge concentrations
Classroom Considerations
- Take a moment to review or introduce Rutherford's Gold Foil Experiment before assigning the interactive
- Allow a little extra time for pupils to make observations at different concentration levels; the interactions happen quickly and cannot be slowed or paused
Pros
- Changing the charge concentration shown in the resource allows for different views of the resulting electric field and directions of force
- The interactive is versatile and has applications in both the chemistry and physics classrooms
Cons
- None