Georgia Department of Education
Ga Virtual Learning: Physical Science: Solutions Chemistry
Through interactive puzzles, informational text and video clips, students will investigate the properties of solutions.
Vision Learning
Visionlearning: Physical States and Properties: Water: Properties and Behavior
Information relating to the properties of water and its behavior.
John Wiley & Sons
Concepts in Biochemistry: Concept Reviews: Water, P H, and Non Covalent Bonding
A detailed review of water and its structure. Included are diagrams, animated graphics, and review quizzes. For the advanced high school student.
CK-12 Foundation
Ck 12: Aqueous Solutions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will define a solution and describe the parts of a solution. They will describe how an aqueous solution is formed from both...
CK-12 Foundation
Ck 12: Aqueous Solutions
[Free Registration/Login may be required to access all resource tools.] In this learning module, students will study water's ability to act as a solvent.
Georgia Department of Education
Ga Virtual Learning: Analysis of Organic Materials Analysis of Organic Materialsv
This comprehensive interactive tutorial continues to explore the forensic science field. Learn how organic materials of evidence are analyzed and what the various types of chromatography are, especially why they are so important to this...
Khan Academy
Khan Academy: Molarity
Learn the definitions of a solution, solute, and solvent. Understand how molarity is used to quantify the concentration of solute, and comcalculations related to molarity.
Khan Academy
Khan Academy: Biology: Water, Acids, and Bases: Solvent Properties of Water
An article investigating why water makes a good solvent. Also, understand what molecules dissolve best in water.
TeachEngineering
Teach Engineering: Chromatography Lab
To increase students' awareness of possible invisible pollutants in drinking water sources, students perform an exciting lab requiring them to think about how solutions and mixtures exist even in unsuspecting places such as ink. They use...
Annenberg Foundation
Annenberg Learner: Virtual Particle Lab: Dissolving
Explore what happens when one substance dissolves into another. Run the simulations and see if you can predict the results.
Concord Consortium
Concord Consortium: Stem Resources: Solubility
Learners can use this web-based activity to study solutions, as far as the intermolecular attractive forces that allow or do not allow a solvent to dissolve in a solute. Also discussed includes the solubility rule "like dissolves like"...
Frostburg State University
Why Does the Solubility of Gases Usually Increase as Temperature Goes Down?
This chemistry course article, "Why does the solubility of gases usually increase as temperature goes down?," provides text and related links on the effects of temperature and pressure on the solubility of gases.
American Chemical Society
Inquiry in Action: Dissolving Different Liquids in Water
This activity will have students experimenting with whether all liquids dissolve in water. Activity includes both teacher and student instructions.
American Chemical Society
Middle School Chemistry: Lesson Plans: Why Does Water Dissolve Salt?
Students use their own model of a salt crystal and water molecule to show how water dissolves salt. Then, they relate their observations to the structure of salt, water, and alcohol on the molecular level.
New York University
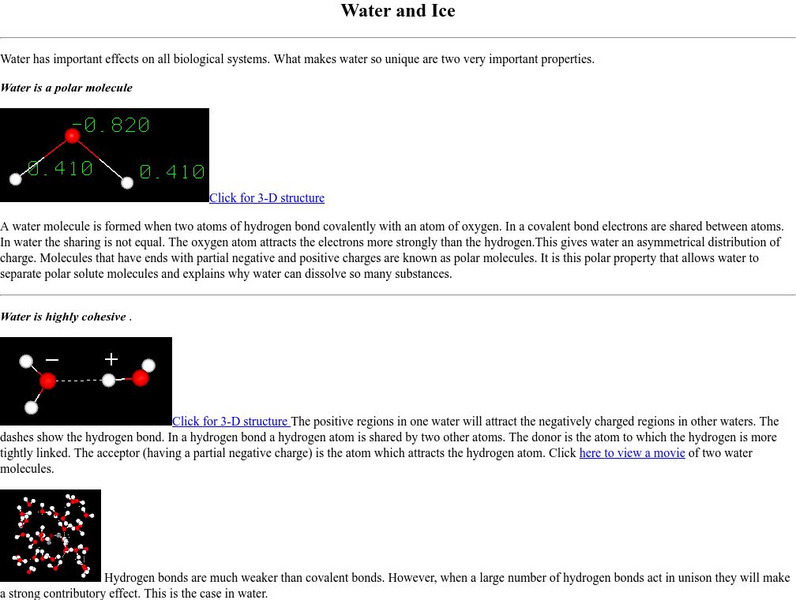
New York University: About Water and Ice
Page uses movies and 3D images to explain how properties of water relate to polarity and hydrogen bonding.
Science Education Resource Center at Carleton College
Serc: Investigating Chromatography: Selecting Variables
In this lab, students will demonstrate observation skills as they design an experiment to separate colors of various water-based pens in order to learn about mixtures and solutions. Students will determine a variable to test and complete...
Crescent Public Schools
The Internet Science Room: Solution Concentration
Students have the opportunity to study examples and worked out example problems to further their understanding of chemical solution concentration.
McGraw Hill
Glencoe Biology: Water and Solutions: Self Check Quiz
Answer five multiple choice questions about water and solutions in this self-checking quiz.
US Geological Survey
Water Properties
This site provides a discussion of the physical properties of water. Click Home to access the site in Spanish.
Concord Consortium
Concord Consortium: Molecular Workbench: Water and Polar Substances
Adjust amounts of ionic charges in this simulation to see how water molecules react to polar substances in solution.
Chem4kids
Chem4 Kids: Making Solutions
What makes up a solution? Are there variables to consider when mixing a solution? Find out with this overview.
CPALMS
Florida State University Cpalms: Florida Students: Hot on the Trail
Learn what affect changing temperature has in regards to chemical reactions.
CK-12 Foundation
Ck 12: Chemistry: Dissolving Process
[Free Registration/Login may be required to access all resource tools.] Describes solution formation and interaction of solute with water.