Curated OER
Unit 3 Bonding
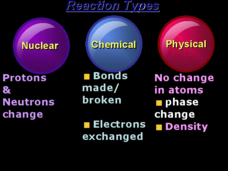
An organized table charting the different types of chemical bonds arrays this resource. The octet rule, ionization energy, and the naming of compounds are also reviewed. Young chemists answer review questions in multiple choice fashion....
Curated OER
Covalent Bonding
Using chlorine as the model to demonstrate the structures and representations, this PowerPoint clearly labels and details the main atomic structures and reasons for bonding. The electrons in the outer shell are diagramed and then the...
Curated OER
Chemistry Slide Collection
A huge slide show provides a review of almost every topic there is to cover in basic chemistry! Your young scientists will be interested to see each illustration and example given. The appearance of the 120 slides varies greatly, most...
Virginia Department of Education
Physical and Chemical Properties of Water
How can you effectively provide detailed concepts of water properties to your high school class in a way they find exciting and challenging at the same time? By letting them play, of course! Through a variety of experiments, pupils...
Curated OER
Chemical Bonding and Shapes of Molecules
In this chemical bonds learning exercise, students review the different types of bonds, Lewis dot structures, ions, and molecule shapes. This learning exercise has 10 matching, 17 multiple choice, and 3 drawing questions.
Cornell University
Atomic Bonding
Explore the connection of surface area to bonding within atoms. Learners complete lab investigations to model changing surface area with different sizes and concentrations of atoms. A flour fireball demonstration follows the labs to...
Royal Society of Chemistry
Investigating Temperature Changes on Evaporating Liquids—Microscale Chemistry
Is there more to evaporation than just less liquid? Show young scientists the energy transformation that occurs during a phase change through a series of simple experiments. Lab partners place drops of water, ethanol, and ethoxyethane on...
Curated OER
Chemical Bonding and Shapes of Molecules
In this chemical bonding learning exercise, students review the different types of bonds and calculate the number of valence electrons in molecules. This learning exercise has 11 matching and 15 multiple choice questions.
Curated OER
Chemistry Foundations
Extensive notes on foundational chemistry concepts make up this resource. It summarizes the properties of matter, the periodic table, chemical nomenclature, and general chemical bonding. Design a set reading comprehension questions to go...
Curated OER
IB Chemistry Practice Test
In this chemistry review worksheet, students practice using Avogadro's number, calculating number of moles, and using the gas laws. This worksheet has 39 multiple choice questions.
NASA
States of Matter
Water, one of the basic needs of humans, is found in all three states of matter on Earth; no other planet—that we know of—possesses this quality. Here is a unit that allows learners to explore through experimentation what it takes to...
Curated OER
2007 U.S. National Chemistry Olympiad Local Section Exam
Sixty multiple choice questions cover the entire gamut of chemistry concepts. This is the local section of the U.S. National Chemistry Olympiad, where your chemistry candidates take a shot at entering the national competition. They...
Curated OER
BioFuels: The Chemistry and Economics of Alternative Fuels
Junior chemists manufacture biodiesel in the lab. In this exercise, they check the purity of the biodiesel using thin layer chromatography. They also calculate its density and heat of combustion. They are sure to rise to the challenge...
American Chemical Society
Forming a Precipitate
Can you mix two liquids to make a solid that is insoluble? Yes, you can, and pupils see this as the lesson uses more than one combination of liquids to form a solid. Through two teacher demonstrations and a hands-on activity, scholars...
US Environmental Protection Agency
Role of Plants in Water Filtration
Investigate the amazing ability of plants to filter contaminants from water with this series of in-class demonstrations. After placing six small, potted plants in plastic cups, different solutions and mixtures are poured into them that...
Science Geek
Intermolecular Forces of Attraction
Chemists love London (dispersion forces)! Presentation begins with an explanation of intermolecular forces including hydrogen bonding, dipole-dipole attraction, and London dispersion forces. It also covers polarity and the relative...
Curated OER
The Effects of Temperature on Chemical Mixtures
Students explore chemical cahnges and the effect of temperature on chemical mixturees, They make observations of the behavior and appearance of certain chemical mixtures and reactions.
Curated OER
Substances And Chemical Reactions
Students watch and predict chemical reactions when different substances are mixed together. They differentiate between solid, liquid, and gas.
Teach Engineering
Density and Miscibility
The liquids did not mix — so what do density columns have to do with it? The seventh part in a series of nine provides the theoretical explanation of why density columns do not mix. The lesson covers the topics related to mixing and...
Cornell University
Unknown Powders
Create a little scientific magic within your classroom! Learners mix powders and liquids and identify chemical reactions. Based on the reactions, individuals determine the identity of various powders.
Royal Society of Chemistry
Energy—Gifted and Talented Chemistry
What has more energy than a room full of pupils after a fire drill? This lesson plan! Explore the changes in energy during different chemical reactions, discover why some reactions feel cold and others feel hot, and tackle the concept of...
Royal Society of Chemistry
State Symbols
When water is a solvent in a chemical equation, we consider it an aqueous solution. Scholars match the name of four states of matter to their proper symbol in a chemical equation. Four puzzles provide repetition to help pupils remember...
Virginia Department of Education
Heat Transfer and Heat Capacity
It's time to increase the heat! Young chemists demonstrate heat transfer and heat capacity in an activity-packed lab, showing the transitions between solid, liquid, and gaseous phases of materials. Individuals plot data as the changes...