Michael Blaber, PhD
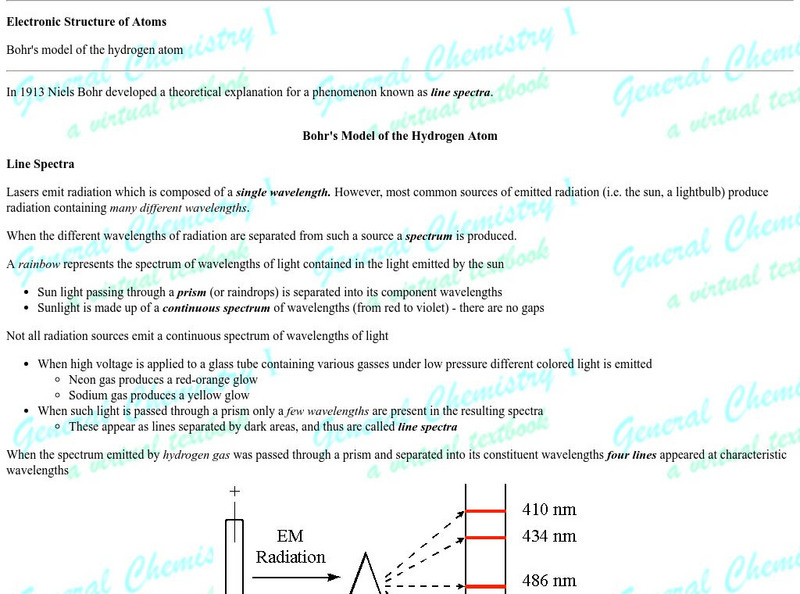
Florida State University: The Bohr Model of the Atom
A well designed clear tutorial explaining the energies involved in the Bohr model of the atom. Illustrations add to the clearly presented equations.
Wyzant
Wyzant: Chem Tutor: Gases
A lengthy page covering most all the gas laws. Each law is described in words and stated as an equation. The use of each law in solving problems is demonstrated. Some problem-solving tips are provided. There are 23 practice problems with...
Wyzant
Wyzant: Reactions
A good resource on chemical reactions, organized by topic: "Examples of chemical changes," "Chemical equations of chemical reactions," "Balancing equations," "Synthesis reactions," "Decomposition reactions," "Single replacement...
Other
Chemistryland: Types of Chemical Reactions Quiz
Seven problem online chemistry quiz that tests understanding of the five categories of chemical reactions.
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] Based on the balanced chemical equation, students will calculate the masses or moles of reactants or products generated in a given reaction. They will also convert...
CK-12 Foundation
Ck 12: Physical Science: Beta Decay
[Free Registration/Login may be required to access all resource tools.] How and why beta decay occurs, its dangers, beta-minus and beta-plus decay and how to write a balanced nuclear equation for beta decay.
CK-12 Foundation
Ck 12: Physical Science: Conservation of Mass in Chemical Reactions
[Free Registration/Login may be required to access all resource tools.] Explains Law of conservation of mass and why chemical equations must be balanced.
Chem4kids
Chem4 Kids: Stoichiometry
This site provides a great overview of stoichiometry, the part of chemistry that studies amounts of substances that are involved in reactions. Content focuses on what you measure, and includes two examples.
Dartmouth College
Dartmouth College: Acids, Bases, and Buffers 1: Monoprotic and Polyprotic Acids
In this experiment, you will explore the behavior of the monoprotic acid (acetic acid) and the polyprotic acid (phosphoric acid). By titrating, you will examine the acid and conjugate base species present across the pH scale and the...
Dartmouth College
Dartmouth College: Chem Lab: Chemical Kinetics 1
In this experiment, you will determine the rate law and confirm the mechanism of the reaction between cyclohexanone and iodine. Requires Java and QuickTime plug-ins to access some features.
Dartmouth College
Dartmouth College: Chem Lab: Chemical Kinetics 2
This lab further analyzes the results of the experiments carried out in Chemical Kinetics 1. In addition, you will carry out experiments to determine the temperature dependence of the reaction rate constant. Requires Java plug-in.
Dartmouth College
Dartmouth College: Chem Lab: Spectra of Conjugated Dyes & Beer's Law
In the first part of this lab, you will measure the absorption spectra of two conjugated dyes and compare the results to the theoretical predictions of a particle-in-a-box model. In the second part of the experiment, you will identify...
Dartmouth College
Dartmouth College: Qualitative Analysis of Anions
"In this experiment, you will observe the reactions of some simple salts, analyze common household chemicals, and identify an unknown sample by testing its reactivity."
Texas Education Agency
Texas Gateway: Conservation of Mass It's the Law!
This resource includes videos, interactives, and additional resources to help students understand the law of conservation of mass and how to balance chemical equations.
Science Education Resource Center at Carleton College
Serc: Chemical Changes: Reacting an Acid and Base
For this chemistry lab, students will investigate chemical changes that occur when acids and bases react. It is meant to introduce the concepts of chemical changes, gases have mass, conservation of mass, and balancing equations. Students...
TED Talks
Ted: Ted Ed: Logarithms Explained
This animated video explores logarithms and explains how they are used in chemistry. Get the basics on these critical mathematical functions -- and discover why smart use of logarithms can determine whether your eyes turn red at the...
Chem Tutor
Chem Tutor: Single Replacement Reactions
Information and examples on single and double replacement reactions, also called single displacement, single substitution, or activity replacement and double displacement, or metathesis. Practice problems with answers are available.
Open Curriculum
Open Curriculum: Introduction to Chemical Stoichiometry
The objective of this article is to teach readers about the basics of chemical stoichiometry.
CK-12 Foundation
Ck 12: Salt Solutions
[Free Registration/Login may be required to access all resource tools.] Students begin by predicting whether a salt solution is acidic, basic, or neutral, and then practice writing balanced equations for hydrolysis reactions. They also...
CK-12 Foundation
Ck 12: Types of Chemical Reactions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will define and give general equations for combination, decomposition, single-replacement, and double-replacement...
CK-12 Foundation
Ck 12: Mole Ratios
[Free Registration/Login may be required to access all resource tools.] In this online tutorial students will relate balanced chemical equations to everyday analogies, such as a recipe. They will also define stoichiometry and use mole...
CK-12 Foundation
Ck 12: Stoichiometric Calculations
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will learn to calculate the amount in moles of a reactant or product from the mass of another reactant or product. They...
CK-12 Foundation
Ck 12: Aqueous Solutions
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will define a solution and describe the parts of a solution. They will describe how an aqueous solution is formed from both...
CK-12 Foundation
Ck 12: Heat
[Free Registration/Login may be required to access all resource tools.] Using the law of conservation of energy as a starting off point, students use the specific heat equation to perform calculations that relate mass, specific heat,...
Other popular searches
- Basic Chemistry Equations
- Chemistry Equations College
- Word Equations Chemistry
- Balance Equation Chemistry
- Chemistry Equations Intro