Fuse School
Quiz: Solids, Liquids and Gases
Time to show what they know! Module five in a 14-part series about solids, liquids, and gases redirects to an interactive quiz. Learners test their skills on topics such as states of matter, phase changes, and Brownian Motion. With...
Curated OER
Periodic Table of Elements
Each of these 111 slides gives the details of one element. The U.S. conventional name, symbol, atomic number and mass, melting point and the boiling point are listed. There are no other details or activities, so this could be used as...
Curated OER
Intermolecular Forces
In this compounds activity, students identify the main intermolecular force in the given compounds and explain the differences between dipole-dipole forces and hydrogen bonds. This activity has 5 short answer questions.
Curated OER
Particle Theory of Matter
In this science word search worksheet, students identify and locate words in a puzzle that relate to particle theory of matter. There are 24 words to locate in the puzzle and spell correctly.
Curated OER
Where There's Smoke......
High schoolers use fundamental relationships between melting points, boiling points, solubility, temperature and pressure to develop explanations. In this chemistry instructional activity students complete an activity.
Mr. E. Science
Changes in Matter
Do solids, liquids, and gases even matter? The presentation focuses on changes in matter, including phases, Boyle's Law, Charles' Law, and physical changes.
Curated OER
Make Your Own Thermometer
Students recognize the concept of temperature, including degrees, and the melting and freezing process. In this 1st - 2nd grade lesson plan, students identify the temperature of various objects, as well as create their own paper...
Curated OER
Density - An Intrinsic Property
Learners discover the property of density while participating in a lab exercise. In this scientific measuring instructional activity, pupils utilize a scale to measure the density of different metal materials. They document their work...
Jefferson Lab
The Periodic Table of Elements
A study of the periodic table of elements doesn't have to be elementary! Deepen understanding of the building blocks of chemistry with an interactive periodic table. At first sight, the table looks like a standard reference page, but a...
Concord Consortium
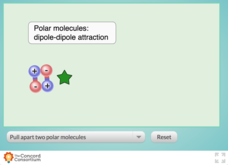
Comparing Dipole-Dipole to London Dispersion
Which intermolecular force is the strongest? Scholars test the relative strength of London dispersion forces, dipole-dipole interactions, and induced dipoles using a simulator. The interactive allows learners to pull on paired molecules...
Science Geek
Thermochemical Calculations
Viewers learn where the heat goes when phase changes take place with a presentation that explains the latent heat of phase changes, or, more specifically, the molar heat of fusion, solidification, vaporization, and condensation. The show...
Curated OER
Melt the Ice
Students examine and discuss how water changes from a liquid to a solid to a gas. They explore this concept by having an ice cube race, competing to see which group can change the solid water back into liquid water first.
Curated OER
Integers and Science Worksheet.
In this science learning exercise, students incorporate their knowledge of math to solve the given problems about freezing points through conversions. They convert from unit to the other. There are 10 questions.
Curated OER
Refrigeration Part 2 - How they work
Fourth graders investigate insulators and how refrigerators actually work to keep things cold through evaporation and pressure changes in the coolant. They create a box to keep an ice cube from melting, and observe a variety of...
Virginia Department of Education
Heat and Thermal Energy Transfer
How does radiation affect our daily lives? Answer that question and others with a lesson that discusses radiation and its use in thermal energy transfer through electromagnetic waves. Pupils investigate vaporization and...
Merck KGaA (Darmstadt, Germany)
EMD PTE
You can't tell by the title, but this is a functional periodic table of elements. Incorporating bright colors, lucid text, and easily operated features, this application serves as a valuable reference tool for your chemistry class.
Curated OER
Snowflake Crystals
Fourth graders explore physical changes and the true life story of Wilson Bentley. They observe the changes that take place with snowflakes. Students discuss what happens when snow flakes melt. They create their own snowflakes using...
Curated OER
State of Matter - Atoms
Learners examine and discuss how atoms change from solid to liquid to gas. They inflate a balloon by melting ice that converts to steam, and discuss the results.
Curated OER
Icebergs Ahead!
Young scholars examine icebergs and how they are suspended in water, why ice floats, the melting process of an iceberg, and the floating behavior of ice compared to that of a cork through a lab activity.
National Energy Education Development Project
Energy Works: Yes, Indeed it Does!
Moving from its definition to how it moves and its different types, scholars see different examples and then move into its application and use in everyday lives, in an energy-based presentation.
Curated OER
Physical Science Review Questions
Prepare your class for a quiz with these physical science review questions. Learners respond to 7 questions about ionic and covalent bonds, oxidation states, and chemical and physical changes. In addition, they name compounds and balance...
Curated OER
Bonding
All different types of bonding are covered in this PowerPoint, along with details of resulting bond and molecule shapes. The definitions of traditional molecule shapes and characteristics of behavior are very useful to assist in...
Curated OER
Alcohols, Phenols, and Ethers
Twelve pages of text tells viewers about the physical properties and naming procedures for alcohols and ethers. A page is also dedicated to briefly describing phenols. This straightforward chemistry presentation comes to you as a PDF...
Curated OER
The Noble Gases
When breaking down the details of the periodic table of elements, this presentation will explain special characteristics for each of the noble gases. The annotations are quite elementary, so this can easily be used in an introductory...