CK-12 Foundation
Ck 12: The Mole Concept
[Free Registration/Login may be required to access all resource tools.] In the following online tutorial students will identify three methods for measuring the amount of matter in a sample. They will define the mole and its relationship...
Frostburg State University
General Chemistry Online: The Mole Concept
Resource provides notes on Moles and Stoichiometry. Deals with all book-keeping aspects, including as section on yields and limiting reactants. Includes lesson plans, lecture slides and notes, links to related websites, and frequently...
Frostburg State University
General Chemistry Online: Faq the Mole Concept
This FAQ site over general chemistry concepts presents the question: "Why are moles used?" It explains what moles are and how they can be used to count molecules and how grams can be turned into moles.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: The Mole Concept
Learn about the chemistry of moles through this slideshow.
Crescent Public Schools
The Internet Science Room: The Mole Concept
Students explore the concept of a mole and molar mass, and how these values are used in chemistry.
Texas Education Agency
Texas Gateway: Mole Conversions
This tutorial reviews over lots of concepts including dimensional analysis, scientific notation, significant figures, and mole conversions.
Upper Canada District School Board
Tom Stretton's Chemistry Pages: The Mole Concept
Deepen your understanding of the mole in chemistry. Find out the origin of the term, and the formulas it represents.
Texas Instruments
Texas Instruments: Mole Activity
This activity is intended to help students gain an understanding of the mole concept. By determining the molar mass of the element at each station, students will be able to identify the element. Students will learn that the molar mass of...
Science Education Resource Center at Carleton College
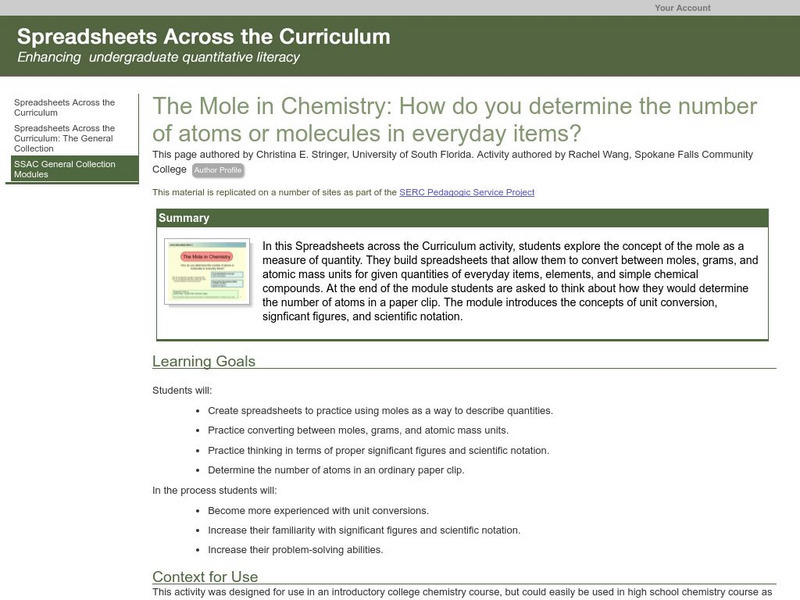
Serc: The Mole in Chemistry: Determining the Number of Atoms in Everyday Items
Students explore the concept of the mole as a measure of quantity. They build spreadsheets that allow them to convert between moles, grams, and atomic mass units for given quantities of everyday items, elements, and simple chemical...
Texas Instruments
Texas Instruments: Mole
This activity is designed to assess the comprehension of concepts related to moles.
Khan Academy
Khan Academy: The Mole and Avogadro's Number
An explanation the mole (named for molecule) and Avogadro's Number. One mole of a substance is equal to 6.022 times 10 to 23rd power units of that substance (such as atoms, molecules, or ions). The number 6.022 time 10 to 23rd power is...
Science and Mathematics Initiative for Learning Enhancement (SMILE)
Smile: Moles, Moles, Moles
This is an activity that is designed to introduce young scholars to the concept of the mole.
Frostburg State University
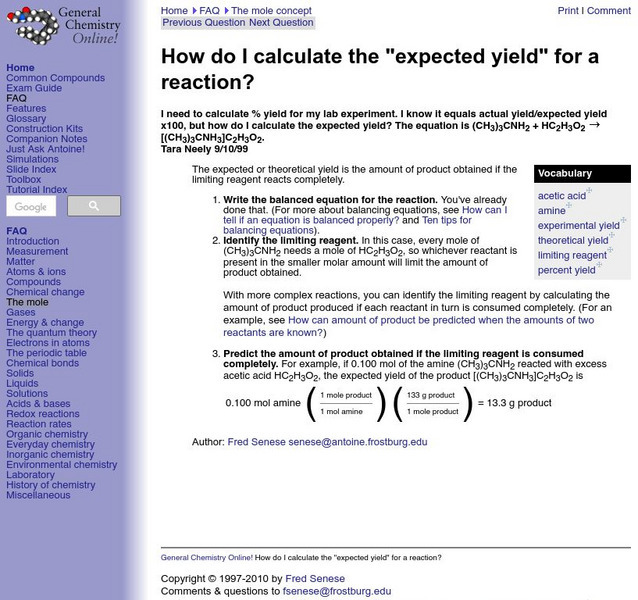
General Chemistry Online: Expected Yield
Frostburg State University provides an example of a problem dealing with how to find expected yield. Includes step-by-step directions on how to solve the problem.
Other
Lock Haven: Glossary of Frequently Misused or Misunderstood Physics Terms
A page, written by Donald E. Simanek of Lock Haven University discusses misused and misunderstood physics terms and concepts. It reminds us that Avogadro's number is a constant, and should be treated as such.
CK-12 Foundation
Ck 12: Flex Book Textbooks: Chemistry Second Edition
[Free Registration/Login may be required to access all resource tools.] A complete, web-based, multi-media textbook covering a wide variety of Chemistry concepts.
Frostburg State University
General Chemistry Online: Limiting Reactant Problem
Explains the essence of limiting reactant problems using a sample problem: How can an amount of product (KNO3) be predicted from amounts for two reactants (KCl and HNO3)? Shows the work and explains how to solve these types of problems.
CK-12 Foundation
Ck 12: Chemistry for High School
This digital textbook covers core chemistry concepts and includes interactive features, real-world examples, and videos.
Science Education Resource Center at Carleton College
Serc: Mn Step: Stoichiometry: Introduction to Stoichiometry
Using the example of a cake recipe, students find the ratios between different ingredients, which introduces the concept of mole ratios. They then calculate the mole ratios in a chemical equation, and learn how this applies to...
Frostburg State University
Frostburg State University: Experimental Yield
Part of a slide show from Frostburg State University showing the concept of experimental yield. Gives reasons for two yields.
Khan Academy
Khan Academy: Molarity vs.osmolarity
Molarity and osmolarity are two distinct concepts. Molarity (M) is the number of moles of solute per liter of solution. The unit of molarity is the mole (mol). Osmolarity (Osm/L) is the total concentration of all solutes in the solution....
Other popular searches
- The Mole Concept
- Explain Mole Concept
- Teaching Mole Concept
- Mole Concept for 9th
- Mole Concept Activities
- Mole Concept 10th Grade
- Mole Concept Question
- Mole Concept With References