Curated by
ACT
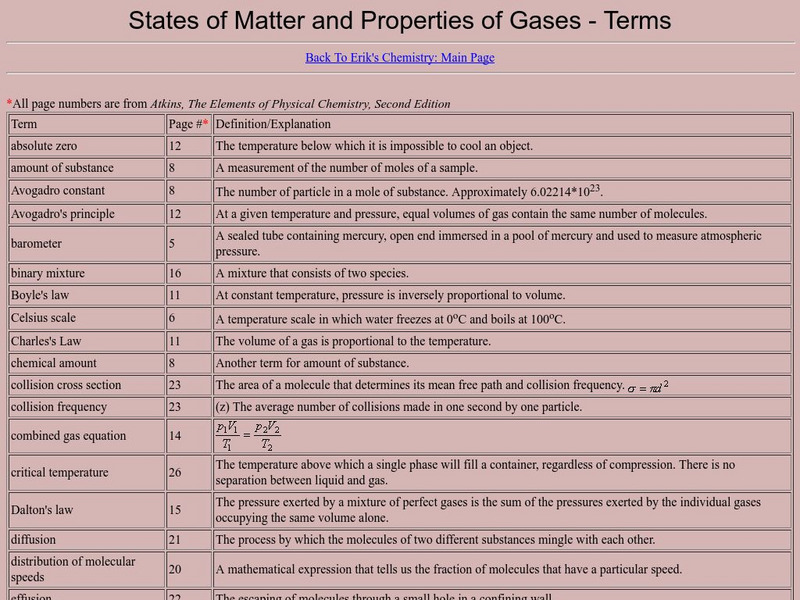
A very complete list of terms that are important to the study of gases. This resource is a web archive.
4 Views
4 Downloads
Concepts
Additional Tags
amount of substance, binary mixture, celsius scale, chemical amount, collision cross section, collision frequency, combined gas equation, critical temperature, distribution of molecular speeds, equation of state, formula unit, gas, gas constant, intermolecular attraction, intermolecular repulsion, isotherm, kinetic theory of gases, limiting law, manometer, mean free path, mechanical equilibrium, perfect gas, perfect gas equation of state, physical chemistry, physical states, property gas, real gas, root-mean-square speed, standard ambient temperature and pressure, standard pressure, state, supercritical fluid, virial coefficients, virial equation of state, avogadro constan, avogadro's principle, charles's law, dalton's law, graham's law of effusion, joule-thompson effect, kelvin scale, linde refrigerator, maxwell distribution of speeds, barometer, kilogram, liquid, mole, mole fraction, solid, van der waals equation, van der waals equation of state, van der waals parameters, van der waals' loops, properties of gas
Classroom Considerations
- Knovation Readability Score: 4 (1 low difficulty, 5 high difficulty)
- This resource is only available on an unencrypted HTTP website.It should be fine for general use, but don’t use it to share any personally identifiable information