Science Geek
Gas Laws
A physical science presentation begins with an explanation of ideal gases and their behavior. Then it introduces all of the gas laws with descriptions and formulas.
Curated OER
States of Matter
Although the title is States of Matter, this presentation is a collection of 4 slides just dealing with gas particle behavior, pressure and the Laws of Boyles and Charles and Gay-Lussac.
Curated OER
Vocabulary - The Gas Laws
In this gas laws activity, students use textbooks and dictionaries to find the definitions of 20 terms associated with the gas laws.
Curated OER
Boyle's Law and Charles's Law
For this gas laws worksheet, students complete 11 fill in the blanks and problem solving questions on Boyle's and Charles's laws.
Curated OER
Boyle's Law Lab
In this gas lab, students use a pressurized soda bottle with a closed end syringe to simulate Boyle's Law. They alter the volume and watch the effects on the pressure of the gas. They plot their data and answer eight post lab questions.
Curated OER
Ideal Gas Problems
In this ideal gas worksheet, students review Boyle's law, Charles's law, and Avogadro's principle. Students use this information to complete 3 problems.
Curated OER
Charles’s Law
Pupils describe the relationship between temperature and volume. In this chemistry lesson, students perform an experiment and record their their results. They use Charles' law to explain their observations.
National Institute of Open Schooling
The Gaseous State
Sixth in a series of 36, this lesson focuses on gases and their behavior in given situations. Learners review the states of matter and then focus on gases, specifically learning Boyle's, Charles's, Avogadro's Laws, Dalton's, and Graham's...
Curated OER
What are Alternative Fuels? -- Gas Laws in Action/Propane Investigation
Students identify the physical properties of propane by applying the gas laws to them. They compare and contrast Charles's and Boyle's gas laws and complete problems using them. They discuss their answers to end the lesson.
Concord Consortium
Concord Consortium: Stem Resources: Gas Laws
A learning module with built-in interactive features where students can read background information on each gas law, then explore the interrelationships of pressure, temperature, and volume. Covers Boyle's Law, Charles's Law,...
Concord Consortium
Concord Consortium: Gas Laws
In this activity, students study gas laws at a molecular level.
Other
Aus E Tute: Ideal Gas Law
Some general information over ideal gases and the ideal gas law. The page shows you how to calculate the volume and pressure of ideal gas and how to calculate moles and gas temperature.
Other
Atoms in Motion: Noble Gases
The ideal gas law is an empirical law; it's a relationship between the pressure in a system of gases to the volume, the temperature, and the number of gas atoms/molecules in the system. This "law" is valid if the gas atoms/molecules are...
University of Colorado
University of Colorado: Ph Et Interactive Simulations: Gas Properties
Experiment with this simulation to better understand the properties of gas with added variables.
Other
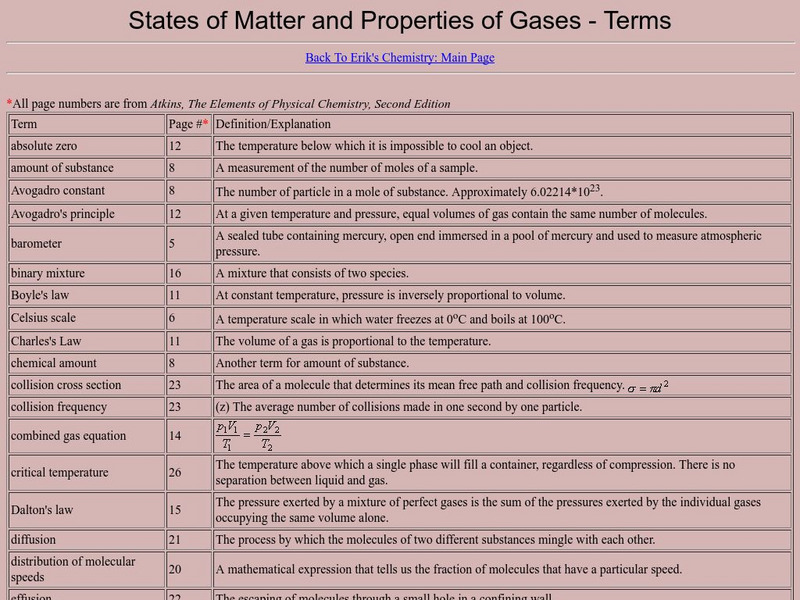
States of Matter and Properties of Gases: Terms
A very complete list of terms that are important to the study of gases. This resource is a web archive.
Other
The Science House: Floating Candles
In this experiment students observe a combustion reaction and deduce the components necessary for the reaction to occur. They also see the relationship between pressure, volume, and number of molecules for gases.