CK-12 Foundation
Ck 12: Life Science: Blood Vessels
[Free Registration/Login may be required to access all resource tools.] The blood vessels are an important part of the cardiovascular system. They connect the heart to every cell in the body. Arteries carry blood away from the heart,...
PBS
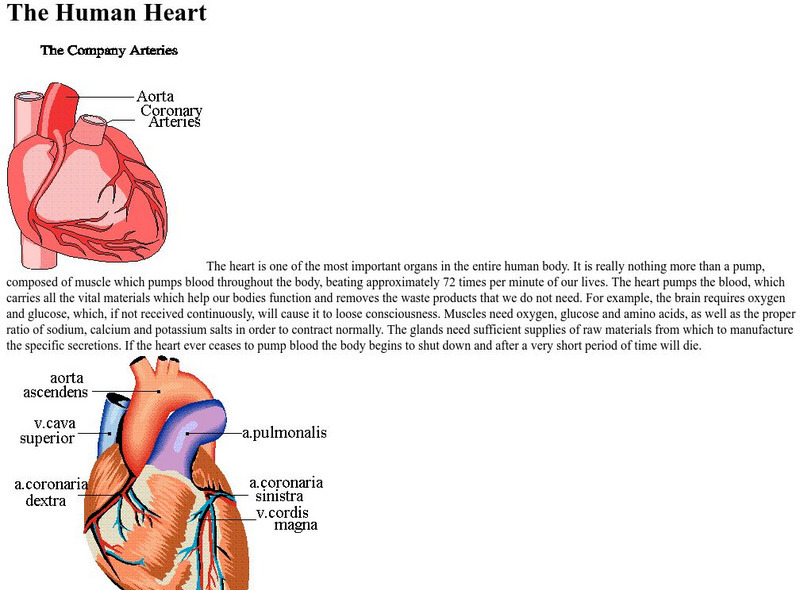
Nova Online: Map of the Human Heart
This PBS site has a concise explanation of how the heart works. There is also a moving diagram of a working heart.
Remedy Health Media
Health Central: Heart Disease
This resource provides an overview about heart disease.
BBC
Bbc: Gcse Bitesize: Cellular Respiration and Transport
The circulatory system transports substances between the exchange surface and cells. It delivers oxygen and glucose to the tissues for respiration, which is the release of energy to cells.
North Central Regional Educational Laboratory
Ncrel: Human Body Systems Resource
What do you know about the different human body systems? This site features information on each of the different systems. Students and teachers will benefit from this comprehensive resource.
North Central Regional Educational Laboratory
Kids learn.org: Circulatory System
What do you know about the circulatory system? This site features several links to help you increase your knowledge of this fascinating human body system.
North Central Regional Educational Laboratory
Circulatory System Internet Workshop
This site features a circulatory system internet workshop focused on diseases and healthy living. Students and teachers will benefit from the resources linked to this site.
Alabama Learning Exchange
Alex: Traffic Jams in the Circulatory System!
Students will discover how atherosclerosis can cause plaque build up in the inner walls of arteries leading to hardening of the arteries which leads to cardiac disease. Students will research atherosclerosis using the Internet and...
CK-12 Foundation
Ck 12: Biology: Circulatory System Study Guide
This comprehensive study guide covers the main terms and concepts needed for a unit on the circulatory system.
Khan Academy
Khan Academy: Circulatory System Questions
This is a ten-question quiz on the Circulatory system.
Khan Academy
Khan Academy: The Effects of High Blood Pressure on the Heart
Read this passage and study the chart to complete a five-question quiz concerning the circulatory system.
Khan Academy
Khan Academy: Chronic Myelogenous Leukemia and the Philadelphia Chromosome
Read this passage and study the graphic to complete the five-question quiz related to the Chronic myelogenous leukemia and Philadelphia Chromosome.
Khan Academy
Khan Academy: Blood Oxygen Levels May Determine Cardiac Muscle Regeneration
Practice for the MCAT by answering questions based on the given bar graphs showing apoptosis and cytokinesis rates.
Khan Academy
Khan Academy: Leukocytes Roll on Blood Vessel Walls
Practice questions related to the circulatory system.
Khan Academy
Khan Academy: Molarity vs.osmolarity
Molarity and osmolarity are two distinct concepts. Molarity (M) is the number of moles of solute per liter of solution. The unit of molarity is the mole (mol). Osmolarity (Osm/L) is the total concentration of all solutes in the solution....
Khan Academy
Khan Academy: Molarity vs. Molality
Learn how molarity and molality differ. The molality of a solution is equal to the moles of solute divided by the mass of solvent in kilograms, while the molarity of a solution is equal to the moles of solute divided by the volume of...
Khan Academy
Khan Academy: The Mole and Avogadro's Number
An explanation the mole (named for molecule) and Avogadro's Number. One mole of a substance is equal to 6.022 times 10 to 23rd power units of that substance (such as atoms, molecules, or ions). The number 6.022 time 10 to 23rd power is...
Khan Academy
Khan Academy: Introduction to Lab Values and Normal Ranges
An introduction to lab values and normal ranges of a blood analysis. A universal model for lab values is explained. Reasons for a value not being with a normal range is discussed.
Khan Academy
Khan Academy: Introduction to Lab Values and Normal Ranges
An explanation of lab values following a blood test using a universal model. Also discussed are reasons why a number may be outside of a normal range. These variances include: age, gender, individual laboratory techniques, and the given...
Khan Academy
Khan Academy: What Is Equivalent?
The equivalent is the amount of a substance which will either react or supply with one mole of hydrogen ions and acid-base reactions or do the same with one mole of electrons in a redox reaction.
Khan Academy
Khan Academy: Units for Common Medical Lab Values
Comparing and contrasting the amount of elements and function (hormone or enzyme activity in lab values.
Khan Academy
Khan Academy: What's Inside Blood?
An explanation of blood from the time it is drawn from the arm to its centrifugation to an analysis of the three components of plasma, white blood cells, and red blood cells.
Khan Academy
Khan Academy: Molarity, Molality, Osmolarity, Osmolality, and Tonicity What's the Difference?
See how each of these terms tells us something different about a solution.