Concord Consortium
Concord Consortium: Stem Resources: Chemical Bonds
By working through this web-based activity, students differentiate between ionic, non-polar covalent, and polar covalent bonds. Specifically, distinctions are made between bonding types based on orbital shapes and electronegativity...
PBS
Pbs Learning Media: Chemical Bonds
This interactive activity developed for Teachers' Domain demonstrates how attractive forces between atoms create chemical bonds, resulting in the formation of molecules and compounds.
Wikimedia
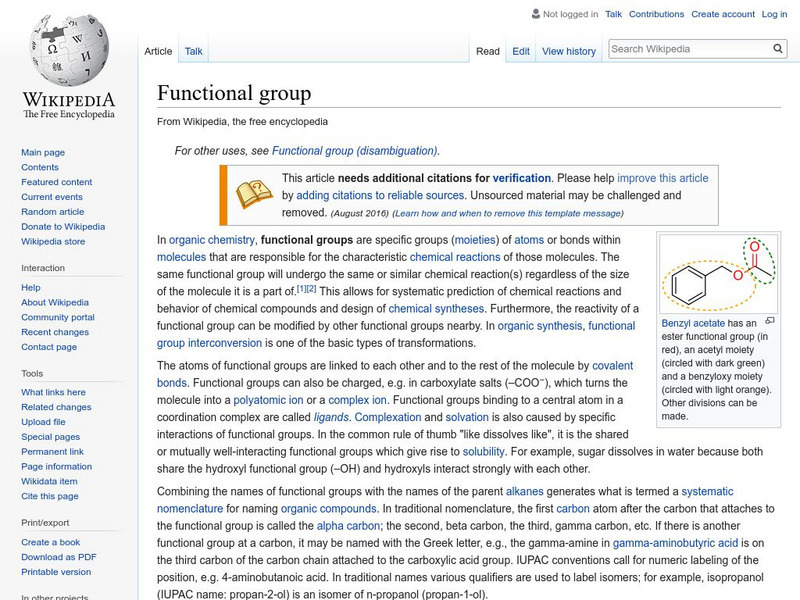
Wikipedia: Functional Group
Encyclopedia entry for functional groups that, in in organic chemistry, are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules.
School of Biological and Chemical Sciences, Queen Mary University of London
Queen Mary University: Glossary
This Queen Mary University site features a glossary of chemical terms, including London forces.
Other
Chemsite: Pure Substances vs. Mixtures
An outline on the topics of pure substances and mixtures.
Nobel Media AB
The Nobel Prize: The Nobel Prize in Chemistry 1934
At this website from The Nobel e-Museum, read about Harold Clayton Urey (1893-1981 CE), the chemist awarded with a Nobel Prize "for his discovery of heavy hydrogen." Download Urey's Nobel Lecture, "Some thermodynamic properties of...
Concord Consortium
Concord Consortium: How Can a Small Spark Start a Huge Explosion?
Students explore connections between electric forces and molecules using simulations, and explain energy transfers using conservation of energy. This concept will be explored in the following activities. Activity 1 What makes materials...
Concord Consortium
Concord Consortium: How Can a Small Spark Start a Huge Explosion?
In this module Activity 3 investigates When atoms get close to each other, what happens to their potential energy? This activity applies energy concepts to compare the potential energy of atoms that are bonded as molecules with the...
Science Education Resource Center at Carleton College
Serc: Charles' Law and Ivory Soap
With this activity students will observe and record the properties of various types of bar soap. They will discover that due to the ingredients some soaps are denser than others, and ivory soap will actually float. Students will see a...
Khan Academy
Khan Academy: The Mole and Avogadro's Number
An explanation the mole (named for molecule) and Avogadro's Number. One mole of a substance is equal to 6.022 times 10 to 23rd power units of that substance (such as atoms, molecules, or ions). The number 6.022 time 10 to 23rd power is...
Chiral Publishing
Chiral Publishing: An Introduction to Chemistry: Molecular Compounds: Audio Book
Learn all about molecular structures in this easy-to-understand, audio book. See how valence electrons determine how atoms bond together, and look at the bonding inside some of the most common molecules around you.
Texas Instruments
Texas Instruments: Matter
This Study Card stack enables students to review the concepts of matter, the kinds and classes of mater including atoms, molecules, elements, compounds, pure substance, and mixtures.
Smithsonian Institution
Smithsonian Science Education Center: Chemical Reactions in Action
This video is a professional development resource that explores student misconceptions about atoms, molecules, and chemical reactions. It also gives some pedagogical approaches for approaching these subjects. [10:12]
University Corporation for Atmospheric Research
Ucar: Nitrogen Oxides
Learn interesting facts about nitrogen oxides, gases whose molecules are made of nitrogen and oxygen atoms and contribute to air pollution.
University Corporation for Atmospheric Research
Ucar: Hydrocarbons
Learn about "hydrocarbons", chemical compounds whose molecules are made up entirely of carbon and hydrogen atoms.
McREL International
Mc Rel: Glue Polymer (Whelmer #15 Learning Activity)
An easy to do activity that investigates the basic principles behind chemical bonding. The activity is written in lesson plan format that meets NSES standards.
Sophia Learning
Sophia: Characteristics of Matter
Find out the basic characteristics and main properties of all matter.
Sophia Learning
Sophia: Converting From Particles to Moles
A guided slide show presentation illustrating how to convert from particles to moles.
Khan Academy
Khan Academy: Stereochemistry Questions
This is a 10-question quiz pertaining to stereochemistry.
Bill Nye
Bill Nye: Hole Y Water
This tutorial from Bill Nye uses an experiment with water and sugar to show how matter is mostly empty space.
Next.cc
Next: Nano Technology
Learn about nanotechnology and why scientists are studying it by completing the three activities. Explore nanotechnology further by clicking on one of the numerous links provided.
Science Education Resource Center at Carleton College
Serc: Plastic Polymers: Investigating Their Flexibility
Young scholars will use their prior knowledge about changes of matter to develop a hypothesis to test the physical properties of materials such as plastic (polymers) and how its chemical properties allow it to have unique physical...
Wikimedia
Wikipedia: Absolute Zero
Wikipedia offers several paragraphs of detailed information on absolute zero, the lowest temperature that can be obtained in any macroscopic system.
Other
Classic Chemistry: Jean Perrin
An excerpt of Perrin's paper "Brownian Motion and Molecular Reality". Includes references.
Other popular searches
- Gumdrop Atoms and Molecules
- Counting Atoms Molecules
- Making Atoms and Molecules
- Atoms & Molecules Ppt
- Matter Atoms Molecules
- Science, Atoms and Molecules
- Science Atoms and Molecules
- Atoms Molecules Elements
- Atoms & Molecules Pot
- Atoms and Molecules Lesson
- Atoms Molecules Units
- Atoms and Molecules Musical